Abstract
Purpose
Long-term follow-up (LTFU) care for childhood cancer survivors (CCSs) is essential to improve and maintain their quality of life. The Survivorship Passport (SurPass) is a digital tool which can aid in the delivery of adequate LTFU care. During the European PanCareSurPass (PCSP) project, the SurPass v2.0 will be implemented and evaluated at six LTFU care clinics in Austria, Belgium, Germany, Italy, Lithuania and Spain. We aimed to identify barriers and facilitators to the implementation of the SurPass v2.0 with regard to the care process as well as ethical, legal, social and economical aspects.
Methods
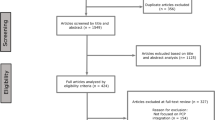
An online, semi-structured survey was distributed to 75 stakeholders (LTFU care providers, LTFU care program managers and CCSs) affiliated with one of the six centres. Barriers and facilitators identified in four centres or more were defined as main contextual factors influencing implementation of SurPass v2.0.
Results
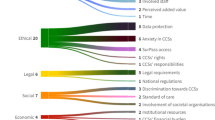
Fifty-four barriers and 50 facilitators were identified. Among the main barriers were a lack of time and (financial) resources, gaps in knowledge concerning ethical and legal issues and a potential increase in health-related anxiety in CCSs upon receiving a SurPass. Main facilitators included institutions’ access to electronic medical records, as well as previous experience with SurPass or similar tools.
Conclusions
We provided an overview of contextual factors that may influence SurPass implementation. Solutions should be found to overcome barriers and ensure effective implementation of SurPass v2.0 into routine clinical care.
Implications for Cancer Survivors
These findings will be used to inform on an implementation strategy tailored for the six centres.


Similar content being viewed by others
Data availability
Study participants did not consent to data sharing outside the PanCareSurPass project. Access to participant data is therefore limited to national and international supervisory authorities. Upon request, the study protocol can be made available (please send an email to s.r.vandenoever-2@prinsesmaximacentrum.nl).
Abbreviations
- CCS:
-
Childhood cancer survivor
- HCP:
-
Healthcare professional
- LTFU:
-
Long-term follow-up
- PCFU:
-
PanCareFollowUp
- PCSP:
-
PanCareSurPass
- SCP:
-
Survivorship care plan
- SurPass:
-
Survivorship Passport
- TS:
-
Treatment summary
References
Steliarova-Foucher E, Fidler MM, Colombet M, Lacour B, Kaatsch P, Pineros M, et al. Changing geographical patterns and trends in cancer incidence in children and adolescents in Europe, 1991-2010 (Automated Childhood Cancer Information System): a population-based study. Lancet Oncol. 2018;19(9):1159–69.
Gatta G, Botta L, Rossi S, Aareleid T, Bielska-Lasota M, Clavel J, et al. Childhood cancer survival in Europe 1999-2007: results of EUROCARE-5--a population-based study. Lancet Oncol. 2014;15(1):35–47.
van Deuren S, Penson A, van Dulmen-den Broeder E, Grootenhuis MA, van der Heiden-van der Loo M, Bronkhorst E, et al. Prevalence and risk factors of cancer-related fatigue in childhood cancer survivors: A DCCSS. LATER study. Cancer. 2022;128(5):1110-1121.
Christen S, Roser K, Mulder RL, Ilic A, Lie HC, Loonen JJ, et al. Recommendations for the surveillance of cancer-related fatigue in childhood, adolescent, and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. J Cancer Surviv. 2020;14(6):923–38.
Mulder RL, Font-Gonzalez A, Hudson MM, van Santen HM, Loeffen EAH, Burns KC, et al. Fertility preservation for female patients with childhood, adolescent, and young adult cancer: recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2021;22(2):e45–56.
Mulder RL, Font-Gonzalez A, Green DM, Loeffen EAH, Hudson MM, Loonen J, et al. Fertility preservation for male patients with childhood, adolescent, and young adult cancer: recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2021;22(2):e57–67.
Norsker FN, Rechnitzer C, Andersen EW, Linnet KM, Kenborg L, Holmqvist AS, et al. Neurologic disorders in long-term survivors of neuroblastoma - a population-based cohort study within the Adult Life after Childhood Cancer in Scandinavia (ALiCCS) research program. Acta Oncol. 2020;59(2):134–40.
Armenian SH, Hudson MM, Mulder RL, Chen MH, Constine LS, Dwyer M, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16(3):e123–36.
Bardi E, Mulder RL, van Dalen EC, Bhatt NS, Ruble KA, Burgis J, et al. Late hepatic toxicity surveillance for survivors of childhood, adolescent and young adult cancer: Recommendations from the international late effects of childhood cancer guideline harmonization group. Cancer Treat Rev. 2021;100:102296.
Teepen JC, van Leeuwen FE, Tissing WJ, van Dulmen-den BE, van den Heuvel-Eibrink MM, van der Pal HJ, et al. Long-term risk of subsequent malignant neoplasms after treatment of childhood cancer in the DCOG LATER Study Cohort: Role of chemotherapy. J Clin Oncol. 2017;35(20):2288–98.
Friend AJ, Feltbower RG, Hughes EJ, Dye KP, Glaser AW. Mental health of long-term survivors of childhood and young adult cancer: A systematic review. Int J Cancer. 2018;143(6):1279–86.
Frederiksen LE, Mader L, Feychting M, Mogensen H, Madanat-Harjuoja L, Malila N, et al. Surviving childhood cancer: A systematic review of studies on risk and determinants of adverse socioeconomic outcomes. Int J Cancer. 2019;144(8):1796–823.
Signorelli C, Wakefield CE, Fardell JE, Wallace WHB, Robertson EG, McLoone JK, et al. The impact of long-term follow-up care for childhood cancer survivors: A systematic review. Crit Rev Oncol Hematol. 2017;114:131–8.
Michel G, Mulder RL, van der Pal HJH, Skinner R, Bardi E, Brown MC, et al. Evidence-based recommendations for the organization of long-term follow-up care for childhood and adolescent cancer survivors: A report from the PanCareSurFup Guidelines Working Group. J Cancer Surviv. 2019;13(5):759–72.
Baird H, Patterson P, Medlow S, Allison KR. Understanding and improving survivorship care for adolescents and young adults with cancer. J Adolesc Young Adult Oncol. 2019;8(5):581–6.
Essig S, Skinner R, von der Weid NX, Kuehni CE, Michel G. Follow-up programs for childhood cancer survivors in Europe: A questionnaire survey. PLoS One. 2012;7(12):e53201.
Haupt R, Essiaf S, Dellacasa C, Ronckers CM, Caruso S, Sugden E, et al. The 'Survivorship Passport' for childhood cancer survivors. Eur J Cancer. 2018;102:69–81.
van Kalsbeek RJ, van der Pal HJH, Hjorth L, Winther JF, Michel G, Haupt R, et al. The European multistakeholder PanCareFollowUp project: Novel, person-centred survivorship care to improve care quality, effectiveness, cost-effectiveness and accessibility for cancer survivors and caregivers. Eur J Cancer. 2021;153:74–85.
Carroll AB. Corporate social responsibility: evolution of a definitional construct. Bus Soc. 1999;38(3):268–95.
de Beijer IAE, Trollip J, van den Oever SR, van Dalen EC, Levitt G, et al. Barriers and facilitators of implementing long-term follow-up care for childhood, adolescent, and young adult cancer survivors: A systematic literature review. Unpublished.
Vaismoradi M, Jones J, Turunen H, Snelgrove S. Theme development in qualitative content analysis and thematic analysis. J Nurs Educ Pract. 2016;6(5):100–10.
de Beijer IAE, van den Oever SR, Charalambous E, Cangioli G, Balaguer J, et al. Information and Technology (IT)-related barriers and facilitators to implementation of a new European eHealth Solution: the Digital Survivorship Passport (SurPass v2.0). Unpublished.
Dulko D, Pace CM, Dittus KL, Sprague BL, Pollack LA, Hawkins NA, et al. Barriers and facilitators to implementing cancer survivorship care plans. Oncol Nurs Forum. 2013;40(6):575–80.
Salz T, McCabe MS, Onstad EE, Baxi SS, Deming RL, Franco RA, et al. Survivorship care plans: Is there buy-in from community oncology providers? Cancer. 2014;120(5):722–30.
Jefford M, Lotfi-Jam K, Baravelli C, Grogan S, Rogers M, Krishnasamy M, et al. Development and pilot testing of a nurse-led posttreatment support package for bowel cancer survivors. Cancer Nurs. 2011;34(3):E1–10.
Spain PD, Oeffinger KC, Candela J, McCabe M, Ma X, Tonorezos ES. Response to a treatment summary and care plan among adult survivors of pediatric and young adult cancer. J Oncol Pract. 2012;8(3):196–202.
Brothers BM, Easley A, Salani R, Andersen BL. Do survivorship care plans impact patients' evaluations of care? A randomized evaluation with gynecologic oncology patients. Gynecol Oncol. 2013;129(3):554–8.
Nicolaije KA, Ezendam NP, Vos MC, Pijnenborg JM, Boll D, Boss EA, et al. Impact of an automatically generated cancer survivorship care plan on patient-reported outcomes in routine clinical practice: Longitudinal outcomes of a pragmatic, cluster randomized trial. J Clin Oncol. 2015;33(31):3550–3559.
de Rooij BH, Ezendam NPM, Nicolaije KAH, Lodder P, Vos MC, Pijnenborg JMA, et al. Survivorship care plans have a negative impact on long-term quality of life and anxiety through more threatening illness perceptions in gynecological cancer patients: The ROGY care trial. Qual Life Res. 2018;27(6):1533–44.
Hill RE, Wakefield CE, Cohn RJ, Fardell JE, Brierley ME, Kothe E, et al. Survivorship care plans in cancer: A meta-analysis and systematic review of care plan outcomes. Oncologist. 2020;25(2):e351–e72.
Kohl S. European health data space proposal launched. Eur J Hosp Pharm. 2022;29(4):240.
Acknowledgements
We would like to thank all respondents for their participation in this study. In addition, we would like to acknowledge Ramona Tallone (Istituto Giannina Gaslini, Genova, Italy), Andrea Beccaria (Istituto Giannina Gaslini, Genova, Italy), Marisa Correcher (Hospital Universitario y Politécnico La Fe, Valencia, Spain) and Antonio Orduña (Hospital Universitario y Politécnico La Fe, Valencia, Spain) for their contribution to survey development and/or data collection.
Funding
This work was supported by the European Union’s Horizon 2020 research and innovation programme (grant number: 899999). The funding source was not involved in study design, data collection, data analysis, interpretation, writing of the manuscript and the decision to submit this article for publication.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the conception and design of this study and design of the surveys. Ethical approvals were obtained and data were collected by authors affiliated with the six participating institutions. Data were analysed by Selina R. van den Oever, Ismay A. E. de Beijer, Leontien C. M. Kremer, Helena J. H. van der Pal and Saskia M. F. Pluijm, and results were interpreted by all authors. The manuscript was drafted by Selina R. van den Oever and critically revised by all authors. All authors approved the final version of this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Ethical review board or institutional approvals were required and obtained in Belgium (Ethics Committee Research UZ Leuven), Italy (Comitato Etico Regionale della Liguria), Germany (Ethik-Komission Universität zu Lübeck) and Spain (Comité de Ética de la Investigación con medicamentos). Institutional approval for pseudonymised data collection, analysis and storage was granted by the Princess Máxima Center, the Netherlands (Clinical Research Committee Princess Máxima Centrum). This study was performed in line with the principles of the Declaration of Helsinki.
Consent to participate
Written informed consent was obtained from all participants included in this study.
Consent for publication
All responses to the survey were pseudonymised. Participants were informed that by participating in this study, they consent to the publication of their pseudonymised responses.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Helena J. H. van der Pal and Saskia M. F. Pluijm are joint last authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van den Oever, S.R., de Beijer, I.A.E., Kremer, L.C.M. et al. Barriers and facilitators to implementation of the interoperable Survivorship Passport (SurPass) v2.0 in 6 European countries: a PanCareSurPass online survey study. J Cancer Surviv 18, 928–940 (2024). https://doi.org/10.1007/s11764-023-01335-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-023-01335-y