Abstract
Purpose
Chemotherapy-induced peripheral neurotoxicity (CIPN) is a common dose-limiting toxicity of cancer treatment causing functional impairment and impacting quality of life. Effective prevention and treatment of CIPN are lacking, and CIPN risk factors remain ill-defined. Metabolic syndrome and associated conditions have emerged as potential risk factors, due to their high prevalence and independent association with nerve dysfunction. This systematic review aimed to investigate the association between these common metabolic-lifestyle factors and CIPN.
Methods
Searches were undertaken using Medline, Embase, CINAHL, Scopus, and Web of Science databases, with additional studies identified from bibliographic references cited by original and review articles. Articles that analyzed metabolic-lifestyle risk factors associated with CIPN for patients treated with platinum- or taxane-based chemotherapy were included.
Results
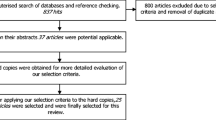
Searches identified 6897 titles; 44 articles had full text review, with 26 studies included. Overall incidence of neuropathy ranged from 16.9 to 89.4%. Obesity had the most consistent patient-oriented evidence as a risk factor for CIPN, with moderate evidence suggesting diabetes did not increase CIPN incidence or severity. A limited number of studies supported an association with low physical activity and greater CIPN risk.
Conclusions
Comorbidities and lifestyle factors, particularly obesity and low physical activity, may contribute to the development of CIPN. The implementation of sensitive outcome measures in large-scale clinical trials is required to further elucidate CIPN risk factors and evaluate if changes in lifestyle would improve long-term CIPN outcomes for cancer survivors.
Implications for Cancer Survivors
Better understanding of CIPN risk profiles may inform personalized medicine strategies and help elucidate pathophysiological mechanisms which could be targeted for neuroprotection.

Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Argyriou AA, Park SB, Islam B, Tamburin S, Velasco R, Alberti P, et al. Neurophysiological, nerve imaging and other techniques to assess chemotherapy-induced peripheral neurotoxicity in the clinical and research settings. J Neurol Neurosurg Psychiatry. 2019. https://doi.org/10.1136/jnnp-2019-320969.
Swain SM, Arezzo JC. Neuropathy associated with microtubule inhibitors: diagnosis, incidence, and management. Clin Adv Hematol Oncol. 2008;6(6):455–67.
Lee JJ, Swain SM. Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(10):1633–42. https://doi.org/10.1200/jco.2005.04.0543.
Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(18):1941–67. https://doi.org/10.1200/jco.2013.54.0914.
Robertson J, Raizer J, Hodges JS, Gradishar W, Allen JA. Risk factors for the development of paclitaxel-induced neuropathy in breast cancer patients. J Peripher Nerv Syst. 2018;23(2):129–33. https://doi.org/10.1111/jns.12271.
Veitch Z, Khan OF, Tilley D, Tang PA, Ribnikar D, Stewart DA, et al. Impact of cumulative chemotherapy dose on survival with adjuvant FEC-D chemotherapy for breast cancer. J Natl Compr Cancer Netw. 2019;17(8):957–67. https://doi.org/10.6004/jnccn.2019.7286.
Hershman DL, Till C, Wright JD, Awad D, Ramsey SD, Barlow WE, et al. Comorbidities and risk of chemotherapy-induced peripheral neuropathy among participants 65 years or older in southwest oncology group clinical trials. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(25):3014–22. https://doi.org/10.1200/JCO.2015.66.2346.
Greenlee H, Hershman DL, Shi Z, Kwan ML, Ergas IJ, Roh JM, et al. BMI, lifestyle factors and taxane-induced neuropathy in breast cancer patients: the Pathways Study. J Natl Cancer Inst. 2017;109(2). https://doi.org/10.1093/jnci/djw206.
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157. https://doi.org/10.1016/j.diabres.2019.107843.
Gérard S. Diabetic neuropathy—a review. Nat Clin Pract Neurol. 2007;3(6):331–40. https://doi.org/10.1038/ncpneuro0504.
Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15(5):288–98. https://doi.org/10.1038/s41574-019-0176-8.
WHO. Obesity and overweight. World Health Organization; 2020. https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight. Accessed Sept 2020.
Davidson EP, Coppey LJ, Calcutt NA, Oltman CL, Yorek MA. Diet-induced obesity in Sprague–Dawley rats causes microvascular and neural dysfunction. Diabetes Metab Res Rev. 2010;26(4):306–18. https://doi.org/10.1002/dmrr.1088.
Callaghan BC, Xia R, Reynolds E, Banerjee M, Rothberg AE, Burant CF, et al. Association between metabolic syndrome components and polyneuropathy in an obese population. JAMA Neurol. 2016;73(12):1468–76. https://doi.org/10.1001/jamaneurol.2016.3745.
IDF. International Diabetes Federation Diabetes Atlas. 9th ed. Brussels, Belgium: International Diabetes Federation; 2019.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41.
Ebell MH, Siwek J, Weiss BD, Woolf SH, Susman J, Ewigman B, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 2004;69(3):548–56.
Barginear M, Dueck AC, Allred JB, Bunnell C, Cohen HJ, Freedman RA, et al. Age and the risk of paclitaxel-induced neuropathy in women with early-stage breast cancer (Alliance A151411): results from 1,881 patients from Cancer and Leukemia Group B (CALGB) 40101. Oncologist. 2019;24(5):617–23. https://doi.org/10.1634/theoncologist.2018-0298.
Furlanetto J, Eiermann W, Marmé F, Reimer T, Reinisch M, Schmatloch S, et al. Higher rate of severe toxicities in obese patients receiving dose-dense (dd) chemotherapy according to unadjusted body surface area: results of the prospectively randomized GAIN study. Ann Oncol. 2016;27(11):2053–9. https://doi.org/10.1093/annonc/mdw315.
Schneider BP, Zhao F, Wang M, Stearns V, Martino S, Jones V, et al. Neuropathy is not associated with clinical outcomes in patients receiving adjuvant taxane-containing therapy for operable breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(25):3051–7. https://doi.org/10.1200/jco.2011.39.8446.
Ottaiano A, Nappi A, Tafuto S, Nasti G, De Divitiis C, Romano C, et al. Diabetes and body mass index are associated with neuropathy and prognosis in colon cancer patients treated with capecitabine and oxaliplatin adjuvant chemotherapy. Oncology. 2016;90(1):36–42. https://doi.org/10.1159/000442527.
Dijksterhuis WPM, Pruijt MJ, Woude SO, Klaassen R, Kurk SA, Oijen MGH, et al. Association between body composition, survival, and toxicity in advanced esophagogastric cancer patients receiving palliative chemotherapy. J Cachexia Sarcopenia Muscle. 2019;10(1):199–206. https://doi.org/10.1002/jcsm.12371.
Alejandro ML, Behrendt EC, Chen EK, Openshaw EH, Shibata ES. Predicting acute and persistent neuropathy associated with oxaliplatin. Am J Clin Oncol. 2013;36(4):331–7. https://doi.org/10.1097/COC.0b013e318246b50d.
Brown JC, Zhang S, Ou F-S, Venook AP, Niedzwiecki D, Lenz H-J, et al. Diabetes and clinical outcome in patients with metastatic colorectal cancer: CALGB 80405 (Alliance). JNCI Cancer Spectrum. 2019;4(1). https://doi.org/10.1093/jncics/pkz078.
Uwah AN, Ackler J, Leighton JC, Pomerantz S, Tester W. The effect of diabetes on oxaliplatin-induced peripheral neuropathy. Clin Colorectal Cancer. 2012;11(4):275–9. https://doi.org/10.1016/j.clcc.2012.05.002.
Ramanathan RK, Rothenberg ML, de Gramont A, Tournigand C, Goldberg RM, Gupta S, et al. Incidence and evolution of oxaliplatin-induced peripheral sensory neuropathy in diabetic patients with colorectal cancer: a pooled analysis of three phase III studies. Ann Oncol. 2010;21(4):754–8. https://doi.org/10.1093/annonc/mdp509.
Molassiotis A, Cheng HL, Leung KT, Li YC, Wong KH, Au JSK, et al. Risk factors for chemotherapy-induced peripheral neuropathy in patients receiving taxane- and platinum-based chemotherapy. Brain Behav. 2019;9(6):e01312. https://doi.org/10.1002/brb3.1312.
Kus T, Aktas G, Kalender ME, Sevinc A, Kul S, Suner A, et al. Taxane-induced peripheral sensorial neuropathy in cancer patients is associated with duration of diabetes mellitus: a single-center retrospective study. Support Care Cancer. 2016;24(3):1175–9. https://doi.org/10.1007/s00520-015-2898-z.
Hershman DL, Unger JM, Crew KD, Till C, Greenlee H, Minasian LM, et al. Two-year trends of taxane-induced neuropathy in women enrolled in a randomized trial of acetyl-L-carnitine (SWOG S0715). J Natl Cancer Inst. 2018;110(6):669–76. https://doi.org/10.1093/jnci/djx259.
Winters-Stone KM, Horak F, Jacobs PG, Trubowitz P, Dieckmann NF, Stoyles S, et al. Falls, functioning, and disability among women with persistent symptoms of chemotherapy-induced peripheral neuropathy. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(23):2604–12. https://doi.org/10.1200/jco.2016.71.3552.
Thomaier L, Jewett P, Brown K, Gotlieb R, Teoh D, Blaes AH, et al. The associations between physical activity, neuropathy symptoms and health-related quality of life among gynecologic cancer survivors. Gynecol Oncol. 2020;158(2):361–5. https://doi.org/10.1016/j.ygyno.2020.05.026.
Stevinson C, Steed H, Faught W, Tonkin K, Vallance JK, Ladha AB, et al. Physical activity in ovarian cancer survivors: associations with fatigue, sleep, and psychosocial functioning. Int J Gynecol Cancer. 2009;19(1):73–8. https://doi.org/10.1111/IGC.0b013e31819902ec.
Simon N, Danso M, Alberico T, Basch E, Bennett A. The prevalence and pattern of chemotherapy-induced peripheral neuropathy among women with breast cancer receiving care in a large community oncology practice. Qual Life Res. 2017;26(10):2763–72. https://doi.org/10.1007/s11136-017-1635-0.
Mols F, Beijers AJ, Vreugdenhil G, Verhulst A, Schep G, Husson O. Chemotherapy-induced peripheral neuropathy, physical activity and health-related quality of life among colorectal cancer survivors from the PROFILES registry. J Cancer Surviv. 2015;9(3):512–22. https://doi.org/10.1007/s11764-015-0427-1.
Griffith KA, Zhu S, Johantgen M, Kessler MD, Renn C, Beutler AS, et al. Oxaliplatin-induced peripheral neuropathy and identification of unique severity groups in colorectal cancer. J Pain Symptom Manag. 2017;54(5):701–6.e1. https://doi.org/10.1016/j.jpainsymman.2017.07.033.
Hsu S-Y, Huang W-S, Lee S-H, Chu T-P, Lin Y-C, Lu C-H, et al. Incidence, severity, longitudinal trends and predictors of acute and chronic oxaliplatin-induced peripheral neuropathy in Taiwanese patients with colorectal cancer. Eur J Cancer Care. 2019;28(2):e12976. https://doi.org/10.1111/ecc.12976.
Petrovchich I, Kober KM, Wagner L, Paul SM, Abrams G, Chesney MA, et al. Deleterious effects of higher body mass index on subjective and objective measures of chemotherapy-induced peripheral neuropathy in cancer survivors. J Pain Symptom Manag. 2019;58(2):252–63. https://doi.org/10.1016/j.jpainsymman.2019.04.029.
Ghoreishi Z, Keshavarz S, Jafarabadi MA, Fathifar Z, Goodman KA, Esfahani A. Risk factors for paclitaxel-induced peripheral neuropathy in patients with breast cancer. BMC Cancer. 2018;18(1):958.
Song S, Min J, Suh S, Jung S, Hahn H, Im S-A, et al. Incidence of taxane-induced peripheral neuropathy receiving treatment and prescription patterns in patients with breast cancer. Support Care Cancer. 2017;25(7):2241–8. https://doi.org/10.1007/s00520-017-3631-x.
Bhatnagar B, Gilmore S, Goloubeva O, Pelser C, Medeiros M, Chumsri S, et al. Chemotherapy dose reduction due to chemotherapy induced peripheral neuropathy in breast cancer patients receiving chemotherapy in the neoadjuvant or adjuvant settings: a single-center experience. Springerplus. 2014;3:366. https://doi.org/10.1186/2193-1801-3-366.
Control CfD, Prevention. National diabetes statistics report, 2020. Atlanta: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020. p. 12–5.
Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. 2013;63(6):419–37. https://doi.org/10.3322/caac.21204.
Rivera DR, Ganz PA, Weyrich MS, Bandos H, Melnikow J. Chemotherapy-associated peripheral neuropathy in patients with early-stage breast cancer: a systematic review. J Natl Cancer Inst. 2017;110(2):131–40. https://doi.org/10.1093/jnci/djx140.
Griffith KA, Merkies IS, Hill EE, Cornblath DR. Measures of chemotherapy-induced peripheral neuropathy: a systematic review of psychometric properties. J Peripher Nerv Syst. 2010;15(4):314–25. https://doi.org/10.1111/j.1529-8027.2010.00292.x.
Postma TJ, Heimans JJ, Muller MJ, Ossenkoppele GJ, Vermorken JB, Aaronson NK. Pitfalls in grading severity of chemotherapy-induced peripheral neuropathy. Ann Oncol. 1998;9(7):739–44. https://doi.org/10.1023/A:1008344507482.
Park SB, Kwok JB, Asher R, Lee CK, Beale P, Selle F, et al. Clinical and genetic predictors of paclitaxel neurotoxicity based on patient- versus clinician-reported incidence and severity of neurotoxicity in the ICON7 trial. Ann Oncol. 2017;28(11):2733–40. https://doi.org/10.1093/annonc/mdx491.
Hertz DL. Concerns regarding use of patient-reported outcomes in biomarker studies of chemotherapy-induced peripheral neuropathy. Pharmacogenomics J. 2019;19:411–6. https://doi.org/10.1038/s41397-019-0093-1.
Lee KM, Jung D, Hwang H, Son KL, Kim TY, Im SA, et al. Pre-treatment anxiety is associated with persistent chemotherapy-induced peripheral neuropathy in women treated with neoadjuvant chemotherapy for breast cancer. J Psychosom Res. 2018;108:14–9. https://doi.org/10.1016/j.jpsychores.2018.02.012.
Callaghan BC, Xia R, Banerjee M, Rekeneire N, Harris TB, Newman AB, et al. Metabolic syndrome components are associated with symptomatic polyneuropathy independent of glycemic status.(Report). Diabetes Care. 2016;39(5):801. https://doi.org/10.2337/dc16-0081.
Callaghan BC, Reynolds E, Banerjee M, Chant E, Villegas-Umana E, Feldman EL. Central obesity is associated with neuropathy in the severely obese. Mayo Clin Proc. 2020;95(7):1342–53. https://doi.org/10.1016/j.mayocp.2020.03.025.
Gallagher D, Visser M, Sepúlveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143(3):228–39. https://doi.org/10.1093/oxfordjournals.aje.a008733.
Gérard S, Bréchemier D, Lefort A, Lozano S, Abellan Van Kan G, Filleron T, et al. Body composition and anti-neoplastic treatment in adult and older subjects - a systematic review. J Nutr Health Aging. 2016;20(8):878–88. https://doi.org/10.1007/s12603-015-0653-2.
Bray GA, Perreault L. Obesity in adults: prevalence, screening, and evaluation. UpToDate Inc.; 2014. https://www.uptodate.com/contents/obesity-in-adults-prevalence-screening-and-evaluation. Accessed Sept 2020.
Li J, Tang J, Li Y, Yu J, Zhang B, Yu C. Pharmacokinetic profile of paclitaxel in the plasma, lung, and diaphragm following intravenous or intrapleural administration in rats. Thorac Cancer. 2015;6(1):43–8. https://doi.org/10.1111/1759-7714.12139.
Ali R, Baracos VE, Sawyer MB, Bianchi L, Roberts S, Assenat E, et al. Lean body mass as an independent determinant of dose-limiting toxicity and neuropathy in patients with colon cancer treated with FOLFOX regimens. Cancer Med. 2016;5(4):607–16. https://doi.org/10.1002/cam4.621.
Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41. https://doi.org/10.1038/s41572-019-0092-1.
Sylvia LG, Bernstein EE, Hubbard JL, Keating L, Anderson EJ. Practical guide to measuring physical activity. J Acad Nutr Diet. 2014;114(2):199–208. https://doi.org/10.1016/j.jand.2013.09.018.
McCrary JM, Goldstein D, Sandler CX, Barry BK, Marthick M, Timmins HC, et al. Exercise-based rehabilitation for cancer survivors with chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2019;27:3849–57. https://doi.org/10.1007/s00520-019-04680-w.
Zimmer P, Trebing S, Timmers-Trebing U, Schenk A, Paust R, Bloch W, et al. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: a randomized controlled trial. Support Care Cancer. 2018;26(2):615–24. https://doi.org/10.1007/s00520-017-3875-5.
Kleckner I, Kamen C, Gewandter J, Mohile N, Heckler C, Culakova E, et al. Effects of exercise during chemotherapy on chemotherapy-induced peripheral neuropathy: a multicenter, randomized controlled trial. Support Care Cancer. 2018;26(4):1019–28. https://doi.org/10.1007/s00520-017-4013-0.
Duregon F, Vendramin B, Bullo V, Gobbo S, Cugusi L, Di Blasio A, et al. Effects of exercise on cancer patients suffering chemotherapy-induced peripheral neuropathy undergoing treatment: a systematic review. Crit Rev Oncol Hematol. 2018;121:90–100. https://doi.org/10.1016/j.critrevonc.2017.11.002.
Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K, Buchanan TA, et al. Effects of aerobic and resistance exercise on metabolic syndrome, sarcopenic obesity, and circulating biomarkers in overweight or obese survivors of breast cancer: a randomized controlled trial. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(9):875–83. https://doi.org/10.1200/JCO.2017.75.7526.
Kanzawa-Lee GA, Larson JL, Resnicow K, Smith EML. Exercise effects on chemotherapy-induced peripheral neuropathy: a comprehensive integrative review. Cancer Nurs. 2020;43(3):E172–e85. https://doi.org/10.1097/ncc.0000000000000801.
Bertin E, Nguyen P, Guenounou M, Durlach V, Potron G, Leutenegger M. Plasma levels of tumor necrosis factor-alpha (TNF-alpha) are essentially dependent on visceral fat amount in type 2 diabetic patients. Diabetes Metab. 2000;26(3):178–82.
You T, Nicklas BJ, Ding J, Penninx BW, Goodpaster BH, Bauer DC, et al. The metabolic syndrome is associated with circulating adipokines in older adults across a wide range of adiposity. J Gerontol A Biol Sci Med Sci. 2008;63(4):414–9. https://doi.org/10.1093/gerona/63.4.414.
Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–15. https://doi.org/10.1038/nri3041.
Li T, Timmins HC, King T, Kiernan MC, Goldstein D, Park SB. Characteristics and risk factors of bortezomib induced peripheral neuropathy: a systematic review of phase III trials. Hematol Oncol. 2020;38(3):229–43. https://doi.org/10.1002/hon.2706.
Funding
This study was supported by a Cancer Institute NSW Program Grant (14/TPG/1-05) and a National Health and Medical Research Council of Australia (NHMRC) Project Grant (#1080521). SBP is supported by an NHMRC Career Development Fellowship (#1148595). MCK was supported by an NHMRC Practitioner Fellowship (1156093) and by ForeFront, a large collaborative research group dedicated to the study of neurodegenerative diseases and funded by the National Health and Medical Research Council of Australia Program Grant (#1132524).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Timmins, H.C., Mizrahi, D., Li, T. et al. Metabolic and lifestyle risk factors for chemotherapy-induced peripheral neuropathy in taxane and platinum-treated patients: a systematic review. J Cancer Surviv 17, 222–236 (2023). https://doi.org/10.1007/s11764-021-00988-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-021-00988-x