Abstract
Purpose
To examine prevalence and predictors of neurocognitive outcomes, social attainment, emotional distress, and health-related quality of life (HRQOL) in long-term survivors of pediatric Wilms tumor (WT).
Methods
One hundred fifty-eight WT survivors (59% female; mean [SD] age 33 [9.1] years; time since diagnosis 29 [9.1] years) and 354 community controls (55.6% female; 35 [10.2] years) completed comprehensive neuropsychological testing and physical examination, including echocardiography/electrocardiography, pulmonary function tests, and endocrine evaluation. Self-report of emotional distress, HRQOL, and social attainment were collected. Impairment was defined in relation to both controls and normative data. Generalized linear models were developed to examine impact of treatment and chronic health conditions on outcomes.
Results
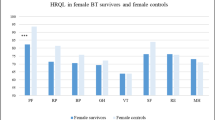
WT survivors performed poorer than norms and controls in 6 of 16 cognitive variables and 1 of 8 HRQOL variables, with scores ranging from − 0.64 (mathematics) to − 0.21 (verbal fluency) standard deviations below expectations. Compared to controls, WT survivors were less likely to graduate college (odds ratio 2.23, 95% confidence interval 1.46–3.41) and had more moderate to severe neurologic conditions (18.4% vs 8.2%, p < 0.001), which were associated with poor memory (β = − 0.90, p < 0.001), attention (β = − 1.02, p < 0.001), and HRQOL general health (β = − 0.80, p = 0.0015). Treatment variables and cardiopulmonary morbidity (higher in survivors) were not associated with outcomes.
Conclusions
Survivors of WT demonstrate impairment in neurocognitive function and have lower social attainment during adulthood, with poorer neurocognitive function associated with neurologic morbidity.
Implications for Cancer Survivors
Survivors of WT should be offered neurocognitive evaluations and rehabilitation. Neurologic conditions should be routinely assessed, and appropriate support offered to reduce risk for functional limitations.

Similar content being viewed by others
References
Noone A, Howlader N, Krapcho M, Miller D, Brest A, Yu M et al. SEER Cancer Statistics Review, 1975-2015, based on November 2017 SEER data submission, posted to the SEER website, April 2018, https://seer.cancer.gov/csr/1975_2015/. Bethesda, MD 2018.
Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. Jama. 2013;309(22):2371–81. https://doi.org/10.1001/jama.2013.6296.
Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61–70. https://doi.org/10.1038/nrc3634.
Smith MA, Seibel NL, Altekruse SF, Ries LA, Melbert DL, O'Leary M, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28(15):2625–34. https://doi.org/10.1200/jco.2009.27.0421.
Robinson KE, Kuttesch JF, Champion JE, Andreotti CF, Hipp DW, Bettis A, et al. A quantitative meta-analysis of neurocognitive sequelae in survivors of pediatric brain tumors. Pediatr Blood Cancer. 2010;55(3):525–31. https://doi.org/10.1002/pbc.22568.
Campbell LK, Scaduto M, Sharp W, Dufton L, Van Slyke D, Whitlock JA, et al. A meta-analysis of the neurocognitive sequelae of treatment for childhood acute lymphocytic leukemia. Pediatr Blood Cancer. 2007;49(1):65–73. https://doi.org/10.1002/pbc.20860.
Cheung YT, Krull KR. Neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia treated on contemporary treatment protocols: a systematic review. Neurosci Biobehav Rev. 2015;53:108–20. https://doi.org/10.1016/j.neubiorev.2015.03.016.
Edelmann MN, Daryani VM, Bishop MW, Liu W, Brinkman TM, Stewart CF, et al. Neurocognitive and patient-reported outcomes in adult survivors of childhood osteosarcoma. JAMA Oncol. 2016;2(2):201–8. https://doi.org/10.1001/jamaoncol.2015.4398.
Krull KR, Sabin ND, Reddick WE, Zhu L, Armstrong GT, Green DM, et al. Neurocognitive function and CNS integrity in adult survivors of childhood Hodgkin lymphoma. J Clin Oncol. 2012;30(29):3618–24. https://doi.org/10.1200/jco.2012.42.6841.
Sleurs C, Deprez S, Emsell L, Lemiere J, Uyttebroeck A. Chemotherapy-induced neurotoxicity in pediatric solid non-CNS tumor patients: an update on current state of research and recommended future directions. Crit Rev Oncol Hematol. 2016;103:37–48. https://doi.org/10.1016/j.critrevonc.2016.05.001.
Scott RH, Stiller CA, Walker L, Rahman N. Syndromes and constitutional chromosomal abnormalities associated with Wilms tumour. J Med Genet. 2006;43(9):705–15. https://doi.org/10.1136/jmg.2006.041723.
Green DM, Kun LE, Matthay KK, Meadows AT, Meyer WH, Meyers PA, et al. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer. 2013;60(7):1083–94. https://doi.org/10.1002/pbc.24487.
Termuhlen AM, Tersak JM, Liu Q, Yasui Y, Stovall M, Weathers R, et al. Twenty-five year follow-up of childhood Wilms tumor: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2011;57(7):1210–6. https://doi.org/10.1002/pbc.23090.
Mostoufi-Moab S, Seidel K, Leisenring WM, Armstrong GT, Oeffinger KC, Stovall M, et al. Endocrine abnormalities in aging survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2016;34(27):3240–7. https://doi.org/10.1200/jco.2016.66.6545.
Buizer AI, de Sonneville LM, van den Heuvel-Eibrink MM, Veerman AJ. Chemotherapy and attentional dysfunction in survivors of childhood acute lymphoblastic leukemia: effect of treatment intensity. Pediatr Blood Cancer. 2005;45(3):281–90. https://doi.org/10.1002/pbc.20397.
Halberg FE, Kramer JH, Moore IM, Wara WM, Matthay KK, Ablin AR. Prophylactic cranial irradiation dose effects on late cognitive function in children treated for acute lymphoblastic leukemia. Int J Radiat Oncol Biol Phys. 1992;22(1):13–6. https://doi.org/10.1016/0360-3016(92)90976-O.
Waber DP, Urion DK, Tarbell NJ, Niemeyer C, Gelber R, Sallan SE. Late effects of central nervous system treatment of acute lymphoblastic leukemia in childhood are sex-dependent. Dev Med Child Neurol. 1990;32(3):238–48. https://doi.org/10.1111/j.1469-8749.1990.tb16930.x.
Meadows A, Massari D, Fergusson J, Gordon J, Littman P, Moss K. Declines in IQ scores and cognitive dysfunctions in children with acute lymphocytic leukaemia treated with cranial irradiation. Lancet. 1981;318(8254):1015–8. https://doi.org/10.1016/S0140-6736(81)91216-2.
Mulhern RK, Kovnar E, Langston J, Carter M, Fairclough D, Leigh L, et al. Long-term survivors of leukemia treated in infancy: factors associated with neuropsychologic status. J Clin Oncol. 1992;10(7):1095–102. https://doi.org/10.1200/jco.1992.10.7.1095.
Mackie E, Hill J, Kondryn H, McNally R. Adult psychosocial outcomes in long-term survivors of acute lymphoblastic leukaemia and Wilms’ tumour: a controlled study. Lancet. 2000;355(9212):1310–4. https://doi.org/10.1016/s0140-6736(00)02112-7.
Lahteenmaki PM, Sankila R, Pukkala E, Kyyronen P, Harila-Saari A. Scholastic achievement of children with lymphoma or Wilms tumor at the end of comprehensive education—a nationwide, register-based study. Int J Cancer. 2008;123(10):2401–5. https://doi.org/10.1002/ijc.23753.
Buizer AI, de Sonneville LM, van den Heuvel-Eibrink MM, Veerman AJ. Behavioral and educational limitations after chemotherapy for childhood acute lymphoblastic leukemia or Wilms tumor. Cancer. 2006;106(9):2067–75. https://doi.org/10.1002/cncr.21820.
Kadan-Lottick NS, Zeltzer LK, Liu Q, Yasui Y, Ellenberg L, Gioia G, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010;102(12):881–93. https://doi.org/10.1093/jnci/djq156.
Barr RD, Chalmers D, De Pauw S, Furlong W, Weitzman S, Feeny D. Health-related quality of life in survivors of Wilms’ tumor and advanced neuroblastoma: a cross-sectional study. J Clin Oncol. 2000;18(18):3280–7. https://doi.org/10.1200/jco.2000.18.18.3280.
Mohrmann C, Henry J, Hauff M, Hayashi RJ. Neurocognitive outcomes and school performance in solid tumor cancer survivors lacking therapy to the central nervous system. J Pers Med. 2015;5(2):83–90. https://doi.org/10.3390/jpm5020083.
Nathan PC, Ness KK, Greenberg ML, Hudson M, Wolden S, Davidoff A, et al. Health-related quality of life in adult survivors of childhood Wilms tumor or neuroblastoma: a report from the childhood cancer survivor study. Pediatr Blood Cancer. 2007;49(5):704–15. https://doi.org/10.1002/pbc.20949.
Elias MF, Dore GA, Davey A. Kidney disease and cognitive function. Contrib Nephrol. 2013;179:42–57. https://doi.org/10.1159/000346722.
Alagiakrishnan K, Mah D, Ahmed A, Ezekowitz J. Cognitive decline in heart failure. Heart Fail Rev. 2016;21(6):661–73. https://doi.org/10.1007/s10741-016-9568-1.
Moon JH. Endocrine risk factors for cognitive impairment. Endocrinol Metab (Seoul). 2016;31(2):185–92. https://doi.org/10.3803/EnM.2016.31.2.185.
Hudson MM, Ness KK, Nolan VG, Armstrong GT, Green DM, Morris EB, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56(5):825–36. https://doi.org/10.1002/pbc.22875.
Hudson MM, Ehrhardt MJ, Bhakta N, Baassiri M, Eissa H, Chemaitilly W, et al. Approach for classification and severity grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomark Prev. 2017;26(5):666–74. https://doi.org/10.1158/1055-9965.Epi-16-0812.
Wechsler D. Wechsler abbreviated scale of intelligence. second ed. San Antonio: Pearson; 2011.
Woodcock RW, McGrew KS, Woodcock Johson NM III. Tests of achievement NU. Rolling Meadows: Riverside Publishing; 2001. p. 2007.
Conners CK. Conners Continuous Performance Test II. North Tonawanda: Multi-Health Systems Inc; 2001.
Delis DC, Kramer JH, Kaplan E, Ober. California Verbal Learning Test - Second Edition. 2 ed. San Antonio, TX. 2000.
Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–14. https://doi.org/10.1016/S0887-6177(03)00039-8.
Reynolds CR, Voress JK. Test of Memory and Learning: Second Edition (TOMAL-II). Austin: Pro-Ed; 2007.
Strauss E, Sherman EMS, Spreen OA. A compendium of neuropsychological test: administration, norms and commentary. 3rd ed. Oxford: Oxford University Press; 2006.
Ware JE Jr. SF-36 health survey update. Spine. 2000;25(24):3130–9.
Derogatis L. Brief Symptom Inventory (BSI): administration, scoring, and procedures manual. Minneapolis: NCS Pearson; 2000.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol. 1995;57(1):289–300.
Bonner MJ, Hardy KK, Willard VW, Gururangan S. Additional evidence of a nonverbal learning disability in survivors of pediatric brain tumors. Children's Health Care. 2009;38:49–63. https://doi.org/10.1080/02739610802615849.
Reddick WE, Russell JM, Glass JO, Xiong X, Mulhern RK, Langston JW, et al. Subtle white matter volume differences in children treated for medulloblastoma with conventional or reduced dose craniospinal irradiation. Magn Reson Imaging. 2000;18(7):787–93.
Askins MA, Moore BD III. Preventing neurocognitive late effects in childhood cancer survivors. J Child Neurol. 2008;23(10):1160–71. https://doi.org/10.1177/0883073808321065.
Perantie DC, Lim A, Wu J, Weaver P, Warren SL, Sadler M, et al. Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatr Diabetes. 2008;9(2):87–95. https://doi.org/10.1111/j.1399-5448.2007.00274.x.
Gaudieri PA, Chen R, Greer TF, Holmes CS. Cognitive function in children with type 1 diabetes. Diabetes Care. 2008;31(9):1892–7. https://doi.org/10.2337/dc07-2132.
Gilchrist LS, Tanner LR, Ness KK. Short-term recovery of chemotherapy-induced peripheral neuropathy after treatment for pediatric non-CNS cancer. Pediatr Blood Cancer. 2017;64(1):180–7. https://doi.org/10.1002/pbc.26204.
Markman M. Chemotherapy-associated neurotoxicity: an important side effect-impacting on quality, rather than quantity, of life. J Cancer Res Clin Oncol. 1996;122(9):511–2. https://doi.org/10.1007/BF01213547.
Mols F, Beijers T, Vreugdenhil G, van de Poll-Franse L. Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support Care Cancer. 2014;22(8):2261–9. https://doi.org/10.1007/s00520-014-2255-7.
Bakitas MA. Background noise: the experience of chemotherapy-induced peripheral neuropathy. Nurs Res. 2007;56(5):323–31. https://doi.org/10.1097/01.Nnr.0000289503.22414.79.
Vileikyte L, Peyrot M, Gonzalez JS, Rubin RR, Garrow AP, Stickings D, et al. Predictors of depressive symptoms in persons with diabetic peripheral neuropathy: a longitudinal study. Diabetologia. 2009;52(7):1265–73. https://doi.org/10.1007/s00125-009-1363-2.
Vileikyte L, Leventhal H, Gonzalez JS, Peyrot M, Rubin RR, Ulbrecht JS, et al. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care. 2005;28(10):2378–83. https://doi.org/10.2337/diacare.28.10.2378.
Shin RK, Stern JW, Janss AJ, Hunter JV, Liu GT. Reversible posterior leukoencephalopathy during the treatment of acute lymphoblastic leukemia. Neurology. 2001;56(3):388–91.
Nazir HF, AlFutaisi A, Zacharia M, Elshinawy M, Mevada ST, Alrawas A, et al. Vincristine-induced neuropathy in pediatric patients with acute lymphoblastic leukemia in Oman: frequent autonomic and more severe cranial nerve involvement. Pediatr Blood Cancer. 2017;64(12). https://doi.org/10.1002/pbc.26677.
Gupta A, Swaroop C, Rastogi R, Garg R, Bakhshi S. Simultaneous occurrence of posterior reversible leukoencephalopathy syndrome in two cases of childhood acute lymphoblastic leukemia induction chemotherapy. Pediatr Hematol Oncol. 2008;25(4):351–8. https://doi.org/10.1080/08880010802016052.
Stott VL, Hurrell MA, Anderson TJ. Reversible posterior leukoencephalopathy syndrome: a misnomer reviewed. Intern Med J. 2005;35(2):83–90. https://doi.org/10.1111/j.1445-5994.2004.00750.x.
Cruz RJ Jr, DiMartini A, Akhavanheidari M, Iacovoni N, Boardman JF, Donaldson J, et al. Posterior reversible encephalopathy syndrome in liver transplant patients: clinical presentation, risk factors and initial management. Am J Transplant. 2012;12(8):2228–36. https://doi.org/10.1111/j.1600-6143.2012.04048.x.
Axt J, Murphy AJ, Seeley EH, Martin CA, Taylor C, Pierce J, et al. Race disparities in Wilms tumor incidence and biology. J Surg Res. 2011;170(1):112–9. https://doi.org/10.1016/j.jss.2011.03.011.
Funding
This study was supported by the National Cancer Institute (CA195547, M. Hudson and L. Robison Principal Investigators). Support to St. Jude Children’s Research Hospital was also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and the American Lebanese Syrian Associated Charities (ALSAC). Neither was involved in the design, analysis, or interpretation of results.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was reviewed and approved by the Institutional Review Board of St Jude Children’s Research Hospital, Memphis, TN, USA. Written consent was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Tonning Olsson, I., Brinkman, T.M., Hyun, G. et al. Neurocognitive outcomes in long-term survivors of Wilms tumor: a report from the St. Jude Lifetime Cohort. J Cancer Surviv 13, 570–579 (2019). https://doi.org/10.1007/s11764-019-00776-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-019-00776-8