Abstract
Purpose
A growing number of research studies have implicated the cerebellum in emotional processing and regulation, especially with regard to negative emotional memories. However, there currently are no studies showing functional changes in the cerebellum as a result of treatment for traumatic stress symptoms. The Neuro Emotional Technique (NET) is an intervention designed to help improve symptoms related to traumatic stress using an integrative approach that combines emotional, cognitive, and motor processing, with a particular focus on autonomic nervous system regulation. In this study, we evaluated whether the NET intervention alters functional connectivity in the brain of patients with traumatic stress symptoms associated with a cancer-related event. We hypothesized that the NET intervention would reduce emotional and autonomic reactivity and that this would correlate with connectivity changes between the cerebellum and limbic structures as well as the brain stem.
Methods
We enrolled patients with a prior cancer diagnosis who experienced distressing cancer-related memories associated with traumatic stress symptoms of at least 6 months in duration. Participants were randomized to either the NET intervention or a waitlist control. To evaluate the primary outcome of neurophysiological effects, all participants received resting-state functional blood oxygen level-dependent (BOLD) magnetic resonance imaging (rs-fMRI) before and after the NET intervention. In addition, autonomic reactivity was measured using heart rate response to the traumatic stimulus. Pre/post comparisons were performed between the NET and control groups.
Results
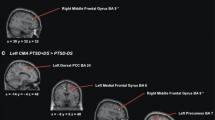
The results demonstrated significant changes in the NET group, as compared to the control group, in the functional connectivity between the cerebellum (including the vermis) and the amygdala, parahippocampus, and brain stem. Likewise, participants receiving the NET intervention had significant reductions in autonomic reactivity based on heart rate response to the traumatic stimulus compared to the control group.
Conclusions
This study is an initial step towards establishing a neurological signature of treatment effect for the NET intervention. Specifically, functional connectivity between the cerebellum and the amygdala and prefrontal cortex appear to be associated with a reduction in autonomic reactivity in response to distressing cancer-related memories.
Implications for cancer survivors
This study contributes to the understanding of possible mechanisms by which interventions like NET may help reduce emotional distress in cancer patients who suffer from traumatic stress symptoms.

Similar content being viewed by others
References
Alter CL, Pelcovitz D, Axelrod A, Goldenberg B, Harris H, Meyers B, et al. Identification of PTSD in cancer survivors. Psychosomatics. 1996;37:137–43.
Meeske KA, Ruccione K, Globe DR, Stuber ML. Posttraumatic stress, quality of life, and psychological distress in young adult survivors of childhood cancer. Oncol Nurs Forum. 2001;28:481–9.
Peres JF, Newberg AB, Mercante JP, Simao M, Albuquerque VE, Peres MJ, et al. Cerebral blood flow changes during retrieval of traumatic memories before and after psychotherapy: a SPECT study. Psychol Med. 2007;37:1–11.
Strata P. The emotional cerebellum. Cerebellum. 2015;14:570–7.
Strata P, Scelfo B, Sacchetti B. Involvement of cerebellum in emotional behavior. Physiol Res. 2011;60(Suppl 1):S39–48.
Yin Y, Li L, Jin C, Hu X, Duan L, Eyler LT, et al. Abnormal baseline brain activity in posttraumatic stress disorder: a resting-state functional magnetic resonance imaging study. Neurosci Lett. 2011;498:185–9.
Baldaçara L, Jackowski AP, Schoedl A, Pupo M, Andreoli SB, Mello MF, et al. Reduced cerebellar left hemisphere and vermal volume in adults with PTSD from a community sample. J Psychiatr Res. 2011;45:1627–33.
Sussman D, Pang EW, Jetly R, Dunkley BT, Taylor MJ. Neuroanatomical features in soldiers with post-traumatic stress disorder. BMC Neurosci. 2016;17:13. https://doi.org/10.1186/s12868-016-0247-x.
Monti DA, Tobia A, Stoner M, Wintering N, Matthews M, He XS, et al. Neuro emotional technique effects on brain physiology in cancer patients with traumatic stress symptoms: preliminary findings. J Cancer Surviv. 2017; https://doi.org/10.1007/s11764-017-0601-8.
Wolpe J. The practice of behavior therapy. New York: Pergamon Press; 1973.
First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders-clinical version (SCID-CV). Washington D.C.: American Psychiatric Press; 1997.
Pitman RK, Scott PO, Forgue DF, de Jong JB, Claiborn JM. Psychophysiologic assessment of post-traumatic stress disorder imagery in Vietnam combat veterans. Arch Gen Psychiatry. 1987;44:970–5.
Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvisi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–56.
Schwartz MS. Biofeedback: a practitioner’s guide. 2nd ed. New York: Guilford; 1995.
Flor H, Miltner W, Birbaumer N. Psychophysiological recording methods. In: Turk DC, Melzack R, editors. Handbook of pain assessment. New York: Guilford; 1992. p. 169–90.
Foa EB, Ehlers A, Clark DM, Tolin DF, Orsillo SM. The posttraumatic cognitions inventory (PTCI): development and validation. Psychol Assess. 1999;11:303–14.
Horowitz MJ, Wilner NR, Alvarez W. Impact of events scale. A measure of subjective stress. Psychosom Med. 1979;41:209–18.
Spielberger CD. Manual for the state trait anxiety inventory: STAI (form Y). Palo Alto CA: Consulting Psychologists Press; 1983.
Derogatis LR. Brief Symptom Inventory 18: Administration Scoring and Procedure Manual. Minnesota. NCS Pearson Inc: Minneapolis; 2001.
Carlson LE, Bultz BD. Cancer distress screening: needs, methods and models. J Psychosom Res. 2003;55:403–9.
Monti DA, Sinnott J, Marchese M, et al. Muscle test comparisons of congruent and incongruent self-referential statements. Percept Mot Skills. 1999;88:1019–28.
Hommel GA. Stagewise rejective multiple test procedure based on a modified Bonferroni test. Biometrika. 1988;5:383–6.
Core Team R. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2016. URL http://www.R-project.org/
Baumann O, Mattingley JB. Functional topography of primary emotion processing in the human cerebellum. NeuroImage. 2012;61:805–11.
Park JY, Gu BM, Kang DH, Shin YW, Choi CH, Lee JM, et al. Integration of cross-modal emotional information in the human brain: an fMRI study. Cortex. 2008;46:161–9.
Ferrucci R, Giannicola G, Rosa M, Fumagalli M, Boggio PS, Hallett M, et al. Cerebellum and processing of negative facial emotions: cerebellar transcranial DC stimulation specifically enhances the emotional recognition of facial anger and sadness. Cognit Emot. 2012;26:786–99.
Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501.
Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–44.
Park JY, Gu BM, Kang DH, Shin YW, Choi CH, Lee JM, et al. Integration of cross-modal emotional information in the human brain: an fMRI study. Cortex. 2008;46:161–9.
Schraa-Tam CK, Rietdijk WJ, Verbeke WJ, Dietvorst RC, van den Berg WE, Bagozzi RP, et al. fMRI activities in the emotional cerebellum: a preference for negative stimuli and goal-directed behavior. Cerebellum. 2012;11:233–45.
Dempesy CW, Tootle DM, Fontana CJ, Fitzjarrell AT, Garey RE, Heath RG. Stimulation of the paleocerebellar cortex of the cat: increased rate of synthesis and release of catecholamines at limbic sites. Biol Psychiatry. 1983;18:127–32.
Marcinkiewicz M, Morcos R, Chretien MCNS. Connections with the median raphe nucleus: retrograde tracing with WGA-apoHRP-gold complex in the rat. J Comp Neurol. 1989;289:11–35.
Utz A, Thürling M, Ernst TM, Hermann A, Stark R, Wolf OT, et al. Cerebellar vermis contributes to the extinction of conditioned fear. Neurosci Lett. 2015;604:173–7.
Supple WF, Kapp BS. The anterior cerebellar vermis: essential involvement in classically conditioned bradycardia in the rabbit. J Neurosci. 1993;13:3705–11.
Supple WF, Leaton RN. Lesions of the cerebellar vermis and cerebellar hemispheres: effects on heart rate conditioning in rats. Behav Neurosci. 1990;104:934–47.
Sacchetti B, Sacco T, Strata P. Reversible inactivation of amygdala, cerebellum, but not perirhinal cortex, impairs reactivated fear memories. Eur J Neurosci. 2007;25:2875–84.
Cacciola A, Milardi D, Calamuneri A, Bonanno L, Marino S, Ciolli P, et al. Constrained spherical deconvolution tractography reveals cerebello-mammillary connections in humans. Cerebellum. 2017;16:483–95.
Blatt GJ, Oblak AL, Schmahmann JD. Cerebellar connections with limbic circuits: anatomy and functional implications. Handbook of the Cerebellum and Cerebellar Disorders. New York: Springer; 2013. p. 479–96.
Seeley WW, Menon V, Schalzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56.
Sang L, Qin W, Liu Y, Zhang Y, Jiang T, Resting-state YC. Functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. NeuroImage. 2012;61:1213–25.
Allen G, McColl R, Barnard H, Ringe WK, Fleckenstein J, Cullum CM. Magnetic resonance imaging of cerebellar-prefrontal and cerebellar-parietal functional connectivity. NeuroImage. 2005;28:39–48.
Silberman EK, Weingartner H. Hemispheric lateralization of functions related to emotion. Brain Cogn. 1986;5:322–53.
Lee GP, Meador KJ, Loring DW, Allison JD, Brown WS, Paul LK, et al. Neural substrates of emotion as revealed by functional magnetic resonance imaging. Cogn Behav Neurol. 2004;17:9–17.
Schienle A, Scharmüller W. Cerebellar activity and connectivity during the experience of disgust and happiness. Neuroscience. 2013;246:375–81.
Hou C, Liu J, Wang K, Li L, Liang M, He Z, et al. Brain responses to symptom provocation and trauma-related short-term memory recall in coal mining accident survivors with acute severe PTSD. Brain Res. 2007;1144:165–74.
Sakamoto H, Fukuda R, Okuaki T, Rogers M, Kasai K, Machida T, et al. Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: a functional MRI study. NeuroImage. 2005;26:813–21.
Stark EA, Parsons CE, Van Hartevelt TJ, Charquero-Ballester M, McManners H, Ehlers A, et al. Post-traumatic stress influences the brain even in the absence of symptoms: a systematic, quantitative meta-analysis of neuroimaging studies. Neurosci Biobehav Rev. 2015;56:207–21.
Buchsbaum MS, Simmons AN, DeCastro A, Farid N, Matthews SC. Clusters of low (18)F-fluorodeoxyglucose uptake voxels in combat veterans with traumatic brain injury and post-traumatic stress disorder. J Neurotrauma. 2015;32:1736–50.
Meng Y, Qiu C, Zhu H, Lama S, Lui S, Gong Q, et al. Anatomical deficits in adult posttraumatic stress disorder: a meta-analysis of voxel-based morphometry studies. Behav Brain Res. 2014;270:307–15.
Funding
This study was funded by the One Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Institutional Review Board of Thomas Jefferson University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent approved by the IRB of Thomas Jefferson University was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Monti, D.A., Tobia, A., Stoner, M. et al. Changes in cerebellar functional connectivity and autonomic regulation in cancer patients treated with the Neuro Emotional Technique for traumatic stress symptoms. J Cancer Surviv 12, 145–153 (2018). https://doi.org/10.1007/s11764-017-0653-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-017-0653-9