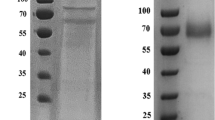
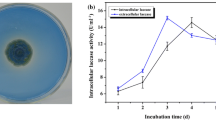
Abstract
Laccase is a copper-containing oxidase enzyme that is present in many microorganisms, plants, and fungi. It plays an important role in the biodegradation of lignin and is known as a lignin-modifying enzyme. Due to the irregular structure of lignin molecules, laccase can utilize a wide range of substrates, such as toxic substances and antibiotics. In this study, laccase from Streptomyces sp. CS29 was studied for properties including optimal pH and temperature, as well as its stability. The effects of some chemicals, metal ions, and organic solvents on laccase activity were also examined. We found that the tested metal ions had different effects on laccase activity. It was observed that 10 and 20 mM Ca2+, 20 mM Zn2+, and 10 mM K+ increased laccase activity, while 10 and 20 mM Fe2+ inhibited it. Laccase activity was also inhibited by 100 mM EDTA and urea but was activated by 50 and 100 mM SDS. Finally, 20% of organic solvents, including ethanol, ethyl acetate, DMSO, acetone, and methanol, decreased laccase activity. Additionally, the crude laccase was tested for sulfamethoxazole degradation. The optimal pH for sulfamethoxazole degradation was pH 3.0 with a 97.90% degradation rate. Furthermore, we used the three-dimensional structure of laccase, obtained from whole genome sequencing data, to investigate the molecular interactions between laccase and sulfamethoxazole. The results of the computational study supported the wet lab experiments in which sulfamethoxazole was degraded by laccase sufficiently. Overall, the results suggest that laccase from Streptomyces sp. CS29 has potential for utilization in bioremediation or environmental biotechnology applications.
Graphical abstract








Similar content being viewed by others
Abbreviations
- ABTS :
-
2,2’-Azino-bis (3-ethylbenzthiazoline-6-sulphonic acid
- DMSO :
-
Dimethyl sulfoxide
- EDTA :
-
Ethylenediaminetetraacetic acid
References
Abdelgalil SA, Attia AR, Reyed RM, Soliman NA (2020) Partial purification and biochemical characterization of a new highly acidic NYSO laccase from Alcaligenes faecalis. J Genet Eng Biotechnol 18(1):79. https://doi.org/10.1186/s43141-020-00088-w
Adnan LA, Sathishkumar P, Yusoff AR, Hadibarata T, Ameen F (2017) Rapid bioremediation of Alizarin Red S and Quinizarine Green SS dyes using Trichoderma lixii F21 mediated by biosorption and enzymatic processes. Bioprocess Biosyst Eng 40(1):85–97. https://doi.org/10.1007/s00449-016-1677-7
Ameen F, Hadi S, Moslem M, Al-Sabri A, Yassin MA (2015a) Biodegradation of engine oil by fungi from mangrove habitat. J Gen Appl Microbiol 61(5):185–192. https://doi.org/10.2323/jgam.61.185
Ameen F, Mohammed M, Hadi S, Al-Sabri AE (2015b) Biodegradation of Low Density Polyethylene (LDPE) by mangrove fungi from the red sea coast. Prog Rubber Plast Recycl Technol 31(2):125–143. https://doi.org/10.1177/147776061503100204
Ameen F, Al-Homaidan AA, Alsamhary K, Al-Enazi NM, AlNadhari S (2021a) Bioremediation of ossein effluents using the filamentous marine cyanobacterium Cylindrospermum stagnale. Environ Pollut 284:117507. https://doi.org/10.1016/j.envpol.2021.117507
Ameen F, Dawoud TM, Alshehrei F, Alsamhary K, Almansob A (2021b) Decolorization of acid blue 29, disperse red 1 and congo red by different indigenous fungal strains. Chemosphere 271:129532. https://doi.org/10.1016/j.chemosphere.2021.129532
Anderson RJ, Weng Z, Campbell RK, Jiang X (2005) Main-chain conformational tendencies of amino acids. Proteins 60(4):679–689. https://doi.org/10.1002/prot.20530
Arregui L, Ayala M, Gomez-Gil X, Gutierrez-Soto G, Hernandez-Luna CE, de Los H, Santos M, Levin L, Rojo-Dominguez A, Romero-Martinez D, Saparrat MCN, Trujillo-Roldan MA, Valdez-Cruz NA (2019) Laccases: structure, function, and potential application in water bioremediation. Microb Cell Fact 18(1):200. https://doi.org/10.1186/s12934-019-1248-0
BIOVIA (2019) Dassault Systèmes, Discovery Studio Visualizer Software, Version 2019 client, San Diego: Dassault Systèmes, 2019. http://www.accelrys.com
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1006/abio.1976.9999
Chen CY, Huang YC, Wei CM, Meng M, Liu WH, Yang CH (2013) Properties of the newly isolated extracellular thermo-alkali-stable laccase from thermophilic actinomycetes, Thermobifida fusca and its application in dye intermediates oxidation. AMB Express 3(1):49. https://doi.org/10.1186/2191-0855-3-49
Chen Y, Stemple B, Kumar M, Wei N (2016) Cell surface display fungal laccase as a renewable biocatalyst for degradation of persistent micropollutants bisphenol A and sulfamethoxazole. Environ Sci Technol 50(16):8799–8808. https://doi.org/10.1021/acs.est.6b01641
Dolinsky TJ, Czodrowski P, Li H, Nielsen JE, Jensen JH, Klebe G, Baker NA (2007) PDB2PQR: expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res 35:W522–W525. https://doi.org/10.1093/nar/gkm276
Dube E, Shareck F, Hurtubise Y, Daneault C, Beauregard M (2008) Homologous cloning, expression, and characterisation of a laccase from Streptomyces coelicolor and enzymatic decolourisation of an indigo dye. Appl Microbiol Biot 79(4):597–603. https://doi.org/10.1007/s00253-008-1475-5
El-NaggarNel A, Abdelwahed NA, Saber WI, Mohamed AA (2014) Bioprocessing of some agro-industrial residues for endoglucanase production by the new subsp.; Streptomyces albogriseolus subsp. cellulolyticus strain NEAE-J. Braz J Microbiol 45(2):743–756. https://doi.org/10.1590/s1517-83822014005000049
Fernandes TAR, da Silveira WB, Passos FML, Zucchi TD (2013) Characterization of a thermotolerant laccase produced by Streptomyces sp. SB086. Ann Microbiol 64(3):1363–1369. https://doi.org/10.1007/s13213-013-0781-z
Ferrandi EE, Spasic J, Djokic L, Vainshtein Y, Senthamaraikannan R, Vojnovic S, Grumaz C, Monti D, Nikodinovic-Runic J (2021) Novel transaminase and laccase from Streptomyces spp. Using combined identification approaches. Catalysts 11(8):919. https://doi.org/10.3390/catal11080919
Gabdulkhakov A, Kolyadenko I, Kostareva O, Mikhaylina A, Oliveira P, Tamagnini P, Lisov A, Tishchenko S (2019) Investigations of accessibility of T2/T3 copper center of two-domain laccase from Streptomyces griseoflavus Ac-993. Int J Mol Sci 20(13):3184. https://doi.org/10.3390/ijms20133184
Gangalla R, Sampath G, Beduru S, Sarika K, KaveriyappanGovindarajan R, Ameen F, Alwakeel S, Thampu RK (2021) Optimization and characterization of exopolysaccharide produced by Bacillus aerophilus rk1 and its in vitro antioxidant activities. J King Saud Univ Sci 33(5):101470. https://doi.org/10.1016/j.jksus.2021.101470
Gaur N, Narasimhulu K, Pydisetty Y (2018) Biochemical and kinetic characterization of laccase and manganese peroxidase from novel Klebsiella pneumoniae strains and their application in bioethanol production. RSC Adv 8(27):15044–15055. https://doi.org/10.1039/c8ra01204k
Gogotya A, Nnolim NE, Digban TO, Okoh AI, Nwodo UU (2021) Characterization of a thermostable and solvent-tolerant laccase produced by Streptomyces sp. LAO. Biotechnol Lett 43(7):1429–1442. https://doi.org/10.1007/s10529-021-03131-z
Govarthanan M, Ameen F, Kamala-Kannan S, Selvankumar T, Almansob A, Alwakeel SS, Kim W (2020) Rapid biodegradation of chlorpyrifos by plant growth-promoting psychrophilic Shewanella sp. BT05: An eco-friendly approach to clean up pesticide-contaminated environment. Chemosphere 247:125948. https://doi.org/10.1016/j.chemosphere.2020.125948
Gunne M, Urlacher VB (2012) Characterization of the alkaline laccase Ssl1 from Streptomyces sviceus with unusual properties discovered by genome mining. PLoS ONE 7(12):e52360. https://doi.org/10.1371/journal.pone.0052360
Haugland JO, Kinney KA, Johnson WH, Camino MMA, Whitman CP, Lawler DF (2019) Laccase removal of 2-chlorophenol and sulfamethoxazole in municipal wastewater. Water Environ Res 91(4):281–291. https://doi.org/10.1002/wer.1006
Huang Y, Yang J (2022) Kinetics and mechanisms for sulfamethoxazole transformation in the phenolic acid-laccase (Trametes versicolor) system. Environ Sci Pollut Res 29(42):62941–62951. https://doi.org/10.1007/s11356-022-20281-3
Janusz G, Pawlik A, Swiderska-Burek U, Polak J, Sulej J, Jarosz-Wilkolazka A, Paszczynski A (2020) Laccase properties, physiological functions, and evolution. Int J Mol Sci 21(3):966. https://doi.org/10.3390/ijms21030966
Jasni MJ, Sathishkumar P, Sornambikai S, Yusoff AR, Ameen F, Buang NA, Kadir MR, Yusop Z (2017) Fabrication, characterization and application of laccase-nylon 6,6/Fe(3+) composite nanofibrous membrane for 3,3’-dimethoxybenzidine detoxification. Bioprocess Biosyst Eng 40(2):191–200. https://doi.org/10.1007/s00449-016-1686-6
Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L, Zhang J, Bolton EE (2021) PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Res 49(D1):D1388–D1395. https://doi.org/10.1093/nar/gkaa971
Li T, Wang HB, Li JW, Jiang LY, Kang HW, Guo ZH, Wang C, Yang W, Liu FF, Lu FP, Liu YH (2021) Enzymatic characterization, molecular dynamics simulation, and application of a novel Bacillus licheniformis laccase. Int J Biol Macromol 167:1393–1405. https://doi.org/10.1016/j.ijbiomac.2020.11.093
Litwińska K, Bischoff F, Matthes F, Bode R, Rutten T, Kunze G (2019) Characterization of recombinant laccase from Trametes versicolor synthesized by Arxula adeninivorans and its application in the degradation of pharmaceuticals. AMB Expr 9(1). https://doi.org/10.1186/s13568-019-0832-3
Machczynski MC, Vijgenboom E, Samyn B, Canters GW (2004) Characterization of SLAC: a small laccase from Streptomyces coelicolor with unprecedented activity. Protein Sci 13(9):2388–2397. https://doi.org/10.1110/ps.04759104
Majumdar S, Lukk T, Solbiati JO, Bauer S, Nair SK, Cronan JE, Gerlt JA (2014) Roles of small laccases from Streptomyces in lignin degradation. Biochemistry 53(24):4047–4058. https://doi.org/10.1021/bi500285t
Mayolo-Deloisa K, Gonzalez-Gonzalez M, Rito-Palomares M (2020) Laccases in food industry: bioprocessing, potential industrial and biotechnological applications. Front Bioeng Biotechnol 8:222. https://doi.org/10.3389/fbioe.2020.00222
Mon WP, Boonlue S, Mongkolthanaruk W (2020) Investigation of Streptomyces for reduction of commercial silk dyes. Korean J Microbiol 56(3):206–213. https://doi.org/10.7845/kjm.2020.0043
Mukhopadhyay M, Banerjee R (2015) Purification and biochemical characterization of a newly produced yellow laccase from Lentinus squarrosulus MR13. 3 Biotech 5(3):227–236. https://doi.org/10.1007/s13205-014-0219-8
Mulla SI, Ameen F, Tallur PN, Bharagava RN, Bangeppagari M, Eqani S, Bagewadi ZK, Mahadevan GD, Yu CP, Ninnekar HZ (2017) Aerobic degradation of fenvalerate by a Gram-positive bacterium, Bacillus flexus strain XJU-4. 3 Biotech 7(5):320. https://doi.org/10.1007/s13205-017-0957-5
Olmeda I, Casino P, Collins RE, Sendra R, Callejon S, Huesa J, Soares AS, Ferrer S, Pardo I (2021) Structural analysis and biochemical properties of laccase enzymes from two Pediococcus species. Microb Biotechnol 14(3):1026–1043. https://doi.org/10.1111/1751-7915.13751
Ouyang B, Xu W, Zhang W, Guang C, Mu W (2022) Efficient removal of sulfonamides and tetracyclines residues by the laccase-mediator system employing a novel laccase from Lysinibacillus fusiformis. J Environ Chem Eng 10(6):108809. https://doi.org/10.1016/j.jece.2022.108809
Patel M, Kumar R, Kishor K, Mlsna T, Pittman CU Jr, Mohan D (2019) Pharmaceuticals of emerging concern in aquatic systems: chemistry, occurrence, effects, and removal methods. Chem Rev 119(6):3510–3673. https://doi.org/10.1021/acs.chemrev.8b00299
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF chimera - A visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612. https://doi.org/10.1002/jcc.20084
Reda FM, El-Mekkawy RM, Hassan NS (2019) Detoxification and bioremediation of sulfa drugs and synthetic dyes by Streptomyces mutabilis A17 laccase produced in solid state fermentation. J Pure Appl Microbio 13(1):85–96. https://doi.org/10.22207/JPAM.13.1.09
Sarnthima R, Khammuang S, Svasti J (2009) Extracellular ligninolytic enzymes by Lentinus polychrous Lév. under solid-state fermentation of potential agro-industrial wastes and their effectiveness in decolorization of synthetic dyes. Biotechnol Bioprocess Eng 14(4):513–522. https://doi.org/10.1007/s12257-008-0262-6
Sathishkumar P, Gu FL, Ameen F, Thayumanavan P (2019) Fungal Laccase Mediated Bioremediation of Xenobiotic Compounds. 1st Edition, 136–158. https://doi.org/10.1201/b22151-8
Selvam K, Ameen F, Amirul Islam M, Sudhakar C, Selvankumar T (2022) Laccase production from Bacillus aestuarii KSK using Borassus flabellifer empty fruit bunch waste as a substrate and assessing their malachite green dye degradation. J Appl Microbiol 133(6):3288–3295. https://doi.org/10.1111/jam.15670
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38(7):3022–3027. https://doi.org/10.1093/molbev/msab120
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461. https://doi.org/10.1002/jcc.21334
Trubitsina LI, Abdullatypov AV, Larionova AP, Trubitsin IV, Alferov SV, Ponamoreva ON, Leontievsky AA (2021) Expression of thermophilic two-domain laccase from Catenuloplanes japonicus in Escherichia coli and its activity against triarylmethane and azo dyes. Peerj 9. https://doi.org/10.7717/peerj.11646
Umar A, Ahmed S (2022) Optimization, purification and characterization of laccase from Ganoderma leucocontextum along with its phylogenetic relationship. Sci Rep 12(1):2416. https://doi.org/10.1038/s41598-022-06111-z
Wang HB, Huang L, Li YZ, Ma JY, Wang S, Zhang YF, Ge XQ, Wang N, Lu FP, Liu YH (2020) Characterization and application of a novel laccase derived from Bacillus amyloliquefaciens. Int J Biol Macromol 150:982–990. https://doi.org/10.1016/j.ijbiomac.2019.11.117
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, Heer FT, de Beer TAP, Rempfer C, Bordoli L, Lepore R, Schwede T (2018) SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res 46(W1):W296–W303. https://doi.org/10.1093/nar/gky427
Wu YR, Luo ZH, Kwok-Kei Chow R, Vrijmoed LL (2010) Purification and characterization of an extracellular laccase from the anthracene-degrading fungus Fusarium solani MAS2. Bioresour Technol 101(24):9772–9777. https://doi.org/10.1016/j.biortech.2010.07.091
Yan H, Xu L, Su J, Wei H, Li X, Cao S (2023) Biotransformation of sulfamethoxazole by newly isolated surfactant-producing strain Proteus mirabilis sp. ZXY4: Removal efficiency, pathways, and mechanisms. Bioresour Technol 385:129422. https://doi.org/10.1016/j.biortech.2023.129422
Yang LH, Qiao B, Xu QM, Liu S, Yuan Y, Cheng JS (2021) Biodegradation of sulfonamide antibiotics through the heterologous expression of laccases from bacteria and investigation of their potential degradation pathways. J Hazard Mater 416:125815. https://doi.org/10.1016/j.jhazmat.2021.125815
Zhang J, Sun L, Zhang H, Wang S, Zhang X, Geng A (2018) A novel homodimer laccase from Cerrena unicolor BBP6: Purification, characterization, and potential in dye decolorization and denim bleaching. PLoS ONE 13(8):e0202440. https://doi.org/10.1371/journal.pone.0202440
Acknowledgements
This research project was financially supported by Thailand Science Research and Innovation (TSRI) 2021 and Mahasarakham University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sarnthima, R., Mongkolthanaruk, W., Sanachai, K. et al. Laccase from Streptomyces sp. CS29 and molecular insight of sulfamethoxazole degradation. Biologia 79, 311–320 (2024). https://doi.org/10.1007/s11756-023-01552-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-023-01552-x