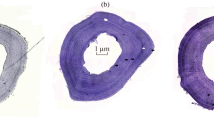
Abstract
The study of growth in amphibians is essential because it directly affects the moment of sexual maturity, timing of reproductive events, fecundity, and longevity. In this study, demographic life-history traits were estimated for the first time in populations of the Lorestan toad (Bufotes luristanicus), living in the southwest of Iran. The microscopic and macroscopic analysis showed that the annual reproductive cycle in B. luristanicus is discontinuous, reaching its peak in March. Metamorphosis was completed in approximately 45 days after spawning, and mean body length at metamorphosis was 14 mm. Significant sexual size dimorphism (SSD) was present in all populations and a larger asymptotic body size was observed 63.78 mm for females vs 55.76 mm for males. The adult survival rate (S) and life expectancy (ESP) were the same for both sexes (S = 0.78 and ESP = 5.05 years). Age and body size were positively correlated with each other for both females and males. Maximum longevity was recorded to be six years in both females and males, and ages of sexual maturity were estimated to be two years in breeding individuals. The adults sample age ranged from two to six (mean age of females: 4.71 ± 0.30 years, in males: 4.37 ± 0.18 years) years. Our data show that females are larger than males and live longer.








Similar content being viewed by others
References
Altunişik A, Özdemir N (2015) Life history traits in Bufotes variabilis (Pallas, 1769) from 2 different altitudes in Turkey. Turk J Zool 39:153–159. https://doi.org/10.3906/zoo-1402-57.10.3906/zoo-1402-57
Amado TF, Bidau CJ, Olalla-Tárraga MÁ (2019) Geographic variation of body size in New World anurans: energy and water in a balance. Ecography 42:456–466. https://doi.org/10.1111/ecog.03889
Bancroft JD, Stevens A (1990) Theory and practice of histological techniques. Churchill Living stone, New York
Begon M, Harper JL, Townsend CR (1996) Ecology: individuals, populations and communities. Blackwell, Oxford
Behera S (2012) Histological observation of gonads during breeding and non breeding season of Trichogaster fasciatus in Shanti Jheel, west Bengal. J Biol 1(6):205–214
Bülbül U, Kutrup B, Eroğlu AI, Koç H, Kurnaz M, Odabaş Y (2018) Life history traits of a Turkish population of the Yellowbellied Toad, Bombina variegata (Linnaeus, 1758) (Anura: Bombinatoridae). Herpetozoa 31(1/2):11–19
Castanet J, Smirina EM (1990) Introduction to the skeletochronological method in amphibians and reptiles. Ann Sci Nat Zool Biol Anim 11:191–196
Castellano S, Giacoma C, Dujsebayeva T (2000) Morphometric and advertisement call geographic variation in polyploidy green toads. Biol J Linn Soc 70:341–360
Chivers DP, Kiesecker J, Marco A, Wildy E, Blaustein AR (1999) Shifts in life history as a response to predation in western toads (Bufo boreas). J Chem Ecol 25(11):2456–2463
Çiçek K, Kumas M, Ayaz D, Mermer A, Engin ŞD (2011) Age structure of Levant water frog, Pelophylax bedriagae, in Lake Sülüklü (Western Anatolia, Turkey). Basic Appl Ecol 25:73–80. https://doi.org/10.11160/bah.11012
Cogâlniceanu D, Miaud C (2003) Population age structure and growth in four syntopic amphibian species inhabiting a large river floodplain. Can J Zool 81:1096–1106. https://doi.org/10.1139/z03-086
Dufresnes C, Mazepa G, Jablonski D et al (2019) Fifteen shades of green: The evolution of Bufotes toads revisited. Mol Phylogen Evol 141:106615. https://doi.org/10.1016/j.ympev.2019.106615
Erismis UC (2018) Age, size, and growth of the Turkish endemic frog Pelophylax caralitanus (Anura: Ranidae). Anat Rec 301:1224–1234. https://doi.org/10.1002/ar.23758
Erismis UC, Chinsamy A (2010) Ontogenetic changes in the epiphyseal cartilage of Rana (Pelophylax) caralitana (Anura: Ranidae). Anat Rec 293:1825–1837. https://doi.org/10.1002/ar.21241
Fakharzadeh F, Darvish J, Kami HG, Ghasemzadeh F, Rastegar-Pouyani E (2014) New karyological and morphometric data on poorly known Bufo surdus and Bufo luristanicus in comparison with data of diploid green toads of the Bufo viridis complex from South of Iran. Asian Herpetol Res 5:168–178
Gibbons MM, McCarthy TK (1983) Age determination of frogs and toads (Amphibia, Anura) from Northwestern Europe. Zool Scr 12(2):145–151
González-Bernal E, Brown GP, Shine CMS, R, (2015) Sex and age differences in habitat use by invasive cane toads (Rhinella marina) and a native anuran (Cyclorana australis) in the Australian wet-dry tropics. Austral Ecol 40(8):953–961. https://doi.org/10.1111/aec.12279
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Guarino FM, Lunardi S, Carlomagno M, Mazzotti S (2003) A skeletochronological study of growth, longevity, and age at sexual maturity in a population of Rana latastei (Amphibia, Anura). J Biosci 28:775–782. https://doi.org/10.1007/BF02708438
Halliday TR, Verrell PA (1988) Body size and age in amphibians and reptiles. J Herpetol 22:253–265. https://doi.org/10.2307/1564148
Hastings A (1997) Population biology: concepts and models. Springer-Verlag, Berlin. https://amphibiaweb.org
Hemelaar A (1985) An improved method to estimate the number of year rings resorbed in phalanges of Bufo bufo (l.) and its implications to populations from different latitudes. Amphibia-Reptilia 6:323–341. https://doi.org/10.1163/156853885X00326
Huang WS, Lin JY, Yu LJU (1996) The male reproductive cycle of the toad, Suto bankorensis, in Taiwan. Zool Stud 35:128–137
IUCN (2014) The IUCN Red List of Threatened Species. Available at http://www.iucnredlist.org/details/54772/0. Accessed 3 Feb 2014
Jin L, Chen CH, Liao W (2017) Altitudinal variation in body size and age in male spot-legged treefrog (Polypedates megacephalus). Russ J Ecol 48:476–481. https://doi.org/10.1134/S1067413617050083
Kaptan E, Murarhanoglu O (2008) Annual morphological cycles of testis and thumb pad of the male frog (Rana ridibunda). Anat Rec 291:1106–1114. https://doi.org/10.1002/ar.20723
Kutrup B, Cakir E, Colak Z, Bulbul U, Karaoglu H (2011) Age and growth of the green toad, Bufo viridis (Laurenti, 1768) from an island and a mainland population in Giresun, Turkey. J Anim Vet Adv 10:1469–1472
Liao WB, Lu X, Shen YW, Hu JC (2011) Age structure and body size of two populations of the rice frog Rana limnocharis from different altitudes. Ital J Zool 78:215–221. https://doi.org/10.1080/11250001003639590
Liao WB, Luo Y, Lou SL, Lu D, Jehle R (2016) Geographic variation in life-history traits: growth season affects age structure, egg size and clutch size in Andrew’s toad (Bufo andrewsi). Front Zool 13:6. https://doi.org/10.1186/s12983-016-0138-0
Lovich JE, Gibbons JW (1992) A review of techniques for quantifying sexual size dimorphism. Growth Develop Aging 56:269–281
McCreary B, Pearl CA, Adams MJ (2008) A protocol for aging anurans using skeletochronology. U.S. Geological Survey open-file report, Virginia
Meshaka WE (2001) The Cuban tree frog in Florida. Life history of a successful colonizing species. University Press of Florida, Gainesville
Morrison C, Hero JM, Browning J (2004) Altitudinal variation in the age at maturity, longevity, and reproductive lifespan of anurans in subtropical Queensland. Herpetologica 60:34–44. https://doi.org/10.1655/02-68
Otero MA, Valetti JA, Bionda CL, Salas NE, Martino AL (2017) Are ploidy and age size-related? A comparative study on tetraploid Pleurodema kriegi and octoploid P. cordobae (Anura: Leptodactylidae) from Central Argentina. Zool Anz 268:136–142. https://doi.org/10.1016/j.jcz.2016.07.005
Patrelle C, Hjernquist MB, Laurila A, Soderman F, Merila J (2012) Sex differences in age structure, growth rate and body size of common frogs Rana temporaria in the subarctic. Pol Biol 35:1505–1513. https://doi.org/10.1007/s00300-012-1190-7
Pesarakloo A, Rastegar Pouyani E, Rastegar-Pouyani N, Najibzadeh M, Khosravani A, Oraie H (2017) The first taxonomic revaluation of the Iranian water frogs of the genus Pelophylax (Anura: Ranidae) using sequences of the mitochondrial genome. Mitochondr DNA 28:392–398. https://doi.org/10.3109/19401736.2015.1127362
Pesarakloo A, Najibzadeh M (2019) Life history of the Levant water frog, Pelophylax bedriagae (Amphibia: Anura: Ranidae) in western Iran. J Anim Divers 1(1):11–19. https://doi.org/10.29252/JAD.2019.1.1.2
Ryser J (1988) Determination of growth and maturation in the common frog, Rana temporaria, by skeletochronology. J Zool 216:673–685. https://doi.org/10.1111/j.1469-7998.tb02465.x
Safaei-Mahroo B, Ghaffari H, Broomand S et al (2015) The herpetofauna of Iran: Checklist of taxonomy, distribution and conservation status. Asian Herpetol Res 6(4):257–290
Seber GAF (1973) The estimation of animal abundance and related parameters. Griffin, London
Sinsch U (2015) Review: Skeletochronological assessment of demographic life-history traits in amphibians. Herpetol J 25:5–13
Sinsch U, Leskovar C, Drobing A, Konig A, Grosse WR (2007) Life-history traits in green toad (Bufo viridis) populations: indicators of habitat quality. Can J Zool 85:665–673. https://doi.org/10.1139/Z07-046
Stöck M, Günther R, Bohme W (2001) Progress towards a taxonomic revision of the Asian Bufo viridis group: Current status of nominal taxa and unsolved problems (Amphibia: Anura: Bufonidae). Zool Abh 51:253–319
Vargas-Salinas F (2006) Sexual size dimorphism in the Cuban tree frog Osteopilus septentrionalis. Amphibia-Reptilia 27:419–426. https://doi.org/10.1163/156853806778189936
Veith M, Kosuch J, Vences M (2003) Climatic oscillations triggered post-messinian speciation of western Palearctic brown frogs (Amphibia, Anura, Ranidae). Mol Phylogen Evol 26:310–327. https://doi.org/10.1016/s1055-7903(02)00324-x
Von Bertalanffy L (1957) Quantitative laws in metabolism and growth. Q Rev Biol 32(3):217–231. https://doi.org/10.1086/401873
Wells KD (2001) The energetics of calling in frogs. In: Ryan ME (ed) Anuran Communication, Smithsonian Press, pp 45–60
Acknowledgements
We thank the honorable authorities of Arak University for helping with the laboratory tasks. We also thank the anonymous reviewers for their careful reading of our manuscript and their valuable comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Toad samples were collected under the 95/10168 permit granted by the Iranian Department of Environment. All the experimental procedures and animal care were performed according to the protocols approved by the Institutional Animal Care and Use Committee of the Arak University), Markazi, Iran (approval cod number: 97/10083061).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pesarakloo, A., Mirkamali, S.J. & Okhovat, S. The first demographic data and life-history trait of Iranian endemic toad, Bufotes luristanicus. Biologia 78, 819–827 (2023). https://doi.org/10.1007/s11756-022-01286-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-022-01286-2