Abstract
Pumpkin (Cucurbita pepo L.) seeds are enriched in bioactive compounds having functional properties. The aim of this study was to analyze the pumpkin seed proteins (PSP) effects on insulin resistance, oxidative stress damage and inflammation in rats with high fructose-induced metabolic syndrome.
Twenty four male Wistar rats, fed isoenergetic diets supplemented with: (1) 20% casein (C); (2) 20% casein and 1 g/kg/day PSP (P); (3) 20% casein and 64% D-fructose (C-HF); (4) 20% casein, 1 g/kg/day PSP and 64% D-fructose (P-HF). After 8 weeks of treatment, fructose supply impaired white adipose tissue (WAT) weight, deteriorated glucose tolerance and tAUC, plasma glucose, insulin, insulinogenic index, HOMA-IR and HOMA-β, antioxidant status, lipid and protein oxidation, plasma TNF-α and IL-6 as compared to control diets. Interestingly, rats assigned to the PSPs diet with or without fructose displayed lower plasma glucose, insulin and fructose, improved tolerance of glucose, tAUC, HOMA-IR and HOMA-β and increased insulinogenic index as compared to C diets. PSPs consumption lowered thiobarbituric acid reactive substances, hydroperoxides and carbonyls in WAT and carbonyls in muscle. Superoxide dismutase and glutathione peroxidase in WAT were significantly diminished in P-HF but increased in P as compared to C-HF and C. Rats fed P-HF diet had low catalase in WAT and high in muscle than those fed C-HF. Moreover, catalase activity increased in muscle but decreased in WAT in P group than in C group. In conclusion, pumpkin seed proteins exhibit favorable effects on metabolic disorders of fructose-induced metabolic syndrome, suggesting a key role in disease therapy.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) is a continuous growing problem, characterized by impaired glucose tolerance, insulin resistance, hypertension, dyslipidaemia, and abdominal adiposity and associated with increased morbidity and mortality (Alberti et al. 2009).
The epidemic intake of refined carbohydrate such as fructose together with saturated fat increased the metabolic syndrome pandemic worldwide. There is consistent data evidence that exposure to excess fructose intake has detrimental effects on multiple cardiometabolic risk factors (Lim et al. 2019; Santos et al. 2019; Stahl et al. 2019).
Although MetS is a significant health problem, the potential involvement of diet, particularly macronutrients, has a major role in the prevention and mitigation of these conditions. In fact, as we have reported in our previous studies, the inclusion of fish protein, omega-3 fatty acids and conjugated linoleic acid into high-fructose diet effectively protects against the development of cardiovascular risk factors in fructose-induced metabolic syndrome rats (Madani et al. 2012, 2015; Mellouk et al. 2012a).
Recently, researchers have paid attention to scientific investigation of dietary plants and herbal preparations as alternative clinical therapies. Among these, Pumpkin plant commonly used as food or medicine (Caili et al. 2006) is an angiosperm belonging to the Cucurbitaceae family. Pumpkin fruit has many nutritional components including pumpkin polysaccharides, active proteins, essential amino acids, carotenoids, and minerals and has been received considerable attentions in recent years because of the nutritional and health protective value of these components (Fokou et al. 2004; Kim et al. 2012). Seeds contain high amount of proteins and fats, ranging from 14.3 to 38.0% and 21.9–54.9%, respectively. In contrast, fruit seems to have relatively low and variable levels of proteins (0.20–23.95%), and a very low content of lipids (0.04–6.57%). Cucurbita, especially the seed parts, can also be a good source of amino acids (Kim et al. 2012). Indeed, among the 20 amino acids which constitute the human proteins, 17 are present in Cucurbita, including the 8 + 1 essential amino acids (Badr et al. 2011; Kim et al. 2012).
To our knowledge, this is the first investigation to explore whether the pumpkin (Cucurbita pepo L.) seeds proteins might be considered for the treatment of metabolic disorders. Cucurbita pepo L. is an interesting target as a compound with prominent pharmacological properties to possible wound healing treatments. Moreover, it has antioxidant and anti-inflammatory effects, antidiabetic, antibacterial and hepato- and chemoprotective properties (Dotto and Chaca 2020). Therefore, the aim of the present study was to further discuss the use and potential benefits of purified pumpkin seed proteins to improve both the metabolic risk factors, such as insulin resistance, glucose tolerance, oxidative stress in tissues, and the inflammation status in rats with high fructose-induced metabolic syndrome.
Materials and methods
Preparation of highly purified pumpkin seed proteins
The proteins, prepared in the laboratory and used in this experiment, was isolated from pumpkin seeds. The seeds of pumpkin (Cucurbita pepo L.) were procured from the local market of Nedroma, Tlemcen, West Algeria. The seeds were well washed with water and dried in the shade. The dried seeds were powdered and the dry powder was stored at 40 °C for further use during experimental study.
The pumpkin seeds powder was diluted with 10 mmol/L of sodium sulfite solution, and homogenizated, and pH was brought to 10 with 1 mmol/L of NaOH. After overnight incubation at 4 °C and under agitation, the homogenate was centrifuged at 3000 g for 20 min at 4 °C. The supernatant was removed and brought to pH 4.5 with 5 mmol/L of H2SO4. After centrifugation, the pellet was dried at 37 °C. The nutrients composition of the purified pumpkin protein was 80 g crude proteins, 8.92 g crude lipids, 1.33 g ashes, 3.36 g moisture, 4.52 g fibers per 100 g protein, with high amounts of arginine, aspartate and glutamic acid, but deficient in lysine and methionine (Table 1).
Experimental design
Twenty four (N = 24) male Wistar rats weighing 190–200 g at the beginning of the study, were purchased from the Animal Research Center, Pasteur Institute Algiers, Algeria, and kept under standard laboratory conditions (temperature of 23 °C, relative humidity of 60% and 12 h of light cycle). Thereafter, animals were randomly divided into four groups of six rats each and, for 8 weeks, were fed isoenergetic diets supplemented with 20% casein (C group) or 20% casein and purified pumpkin seed proteins, administered by oral gavage, at the dose of 1 g/kg/day (P group), or 20% casein enriched with 64% fructose (C-HF group) (Prolabo, Paris, France, 67.19% of total energy), or 20% casein, purified pumpkin seed proteins, administered by oral gavage, at the dose of 1 g/kg/day and enriched with 64% fructose (P-HF group). The composition of the diets is defined in Table 2. Diets given in powdered form, were isocaloric (16.28 MJ/kg) and contained identical amounts of sucrose, lipids, vitamins, minerals and fiber. Food and water were provided ad libitum and were measured daily. The body weight of each animal was recorded once a week. This study was carried out in accordance with the general guidelines for the care and use of laboratory animals (Council of European Communities, 1986).
Intraperitoneal glucose tolerance test
An intraperitoneal glucose tolerance test (IPGTT) was performed at fiftieth day of the experiment. After overnight starvation, the glucose was administered (1.0 g D-glucose/kg body weight) intraperitoneally in the rats as a 50% (w/w) D-glucose solution. Blood samples were collected from the tail vein before (time zero) and 15, 30, 60 and 120 min after the administration of D-glucose. Blood glucose levels were measured by the glucose meter (Accu-Chek Aviva; Roche Diagnostics, Basel, Switzerland). These measurements allowed computing the area under the plasma D-glucose curve (AUC) during the IPGTT.
Blood and tissue collection
At day 60, after a 12-hour fast, rats were sacrificed under general anaesthesia with sodium pentobarbital (60 mg/kg body weight, BW) (Sigma, St Louis, MO, USA). Blood was withdrawn from abdominal aorta and collected into tubes containing Na2-EDTA (0.1%) (Sigma, St Louis, MO, USA). Plasma was separated from blood by low-speed centrifugation at 3000 × g for 20 min at 4 °C and stored at -70˚C in multiple aliquots until analysis. White (WAT) and epididymal (EAT) adipose tissues and muscle were quickly excised, rinsed with a cold saline solution, weighed and immediately frozen at -70˚C until for analysis. The body composition index, considered as an index of adiposity, was calculated as the ratio epididymal fat/gastrocnemius muscle (g/g) (Naderali and Williams 2003).
Plasma analysis
Fasting plasma glucose (Biosystem kit, Barcelona, Spain) and insulin [insulin (rat) ELISA kit, SPI-BIO, Montigny Le Bretonneux, France]were measured at 8 weeks of the experiment. The insulinogenic index (IGI), i.e. the ratio between plasma insulin concentrations and the difference between D-glucose concentration and 4.0 mM (taken as the threshold value for stimulation of insulin secretion by hexose), HOMA-IR index for insulin resistance was calculated using the formula: glycemia (mmol/L) x insulinemia (µU/mL)/22.5). HOMA-βwas calculated according the formula: [20 x insulin (µU/L)]/[fasting glucose (mmo/L) − 3.5]. Plasma fructose levels were quantified enzymatically using a BioSentec Glucose/Fructose/Sucrose kit (BioSentec, Toulouse, France).
WAT and muscle tissues protein and lipid oxidation
WAT and muscle tissue homogenates were prepared on ice in a ratio of 1 g wet tissues to 9 mL of 150 mmol/L KCl using ultraturrax homogenizer. Thiobarbituric acid-reactive substances (TBARS) were measured by the method of Quintanilha et al. (1982), using malondialdehyde as a standard. Tissue lipid hydroperoxide (LHP) levels were evaluated according to Eymand and Genot (2003). Carbonyl concentrations were determined by using Levine et al. (1990) assay. Tissue nitric oxide (NO) was assessed using the Griess reagent (sulfanilamide and Nnaphthyl ethylenediamine) (Cortas and Wakid 1990). Briefly, tissue homogenates were clarified by zinc sulfate solution (Sigma-Aldrich), NO3 (Sigma-Aldrich) was then reduced to NO2 by cadmium (Sigma-Aldrich) overnight at 20˚C under agitation. Samples were added to the Griess reagent and incubated for 20 min at room temperature. The absorbance of these solutions was measured at 540 nm using a Beckman Coulter DU 640 spectrophotometer. Sodium nitrite (Sigma-Aldrich), a strong antioxidant, was used for the standard curve. The data were expressed as µmol/mg proteins.
WAT and muscle tissues antioxidant enzyme activities
WAT and muscle tissue homogenates prepared on ice at a ratio of 1 g wet tissue to 9 mL 150 mmol/L KCl using a POLYTRONPT 2100 homogenizer (Kinematica AG, Lucerne, Switzerland), were used for superoxide dismutase (SOD; EC 1.15.1.1), glutathione peroxidase (GSH-Px; EC 1.11.1.9) and catalase (CAT; EC 1.11.1.6) determinations. Tissue protein concentrations were determined by the method of Lowry et al. (1951), using bovine serum albumin as standard. Tissue SOD activity were determined using Cayman Chemical kit (Cayman Chemical Company, Ann Arbor, MI, USA). Briefly, the method uses xanthine and xanthine oxidase to generate superoxide radicals, which react with 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-phenyl tetrazolium chloride to form a formazan dye. The SOD activity was measured by the degree of inhibition of the reaction, using a spectrophotometer. The results were expressed as U/mg of protein. CAT activity was determined according to the method described by Aebi (1974) and the results were expressed as nmol/mg of protein. Tissue GPH-Px activity was measured using an enzymatic kit (Cayman Chemical Company). The data were expressed in nmol/min/mg of protein.
Plasma tumor necrosis factor and interleukin-6 concentrations
Plasma tumor necrosis factor (TNF-α) and interleukin-6 (IL-6) concentrations were quantified using a commercial kit (RayBiotech, Norcross GA, USA).
Statistical analysis
Data are expressed as means ± SD for continuous variables and percentage for categorical variables. Statistical analysis was performed using the IBM SPSS Statistics 20 Statistical Package for Social Sciences (IBM Software Group, Licensing, 233 S, Wacker Dr, Chicago, IL 60,606, United State). Data were tested using two-way analys of variance (ANOVA) with type of protein and fructose content as independent variables. When the interaction was significant, Student’s least significant difference test was performed. Differences were considered significant at P ≤ 0.05.
Results
Effects on body weight, food intake, weight gain and food efficiency
The body weight, food intake, weight gain, food efficiency, BMI and length are shown in Table 3. The average body weight, BMI and length did not differ between all the groups at the end of a 60-day period. The food intake was much lower in C-HF rats than that recorded in the C rats. After 8 weeks, P-HF rats displayed significant higher body weight gain than P rats. Furthermore, feeding P diet led to low weight gain and food efficiency as judged from the ratio between the relative increase in body weight and mean value for daily food intake than feeding C diet.
Effects on organ weights
Table 4 listed the wet weight of the muscle, white and epididymal adipose tissues and adiposity index. No significant difference was showed in absolute and relative EAT and muscle weights among the groups. The absolute and relative WAT wet weights, however, rose by about 61% and 40%, respectively, in C-HF group relative to C. Interestingly, we found that treatment of HF rats with pumpkin seed proteins reduced by approximately 11% adiposity index without modifying absolute and relative WAT, EAT and muscle weights compared with the C-HF group.
Effects on glucose tolerance test and glucose AUC
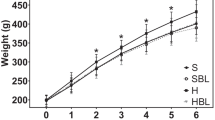
The response of the rats to a physiological glucose challenge was first tested using an IPGTT. Addition of fructose in both protein diets induced significant increased (P < 0.05) in the glucose level at all time points as compared to that in the control rats (Fig. 1). Indeed, glucose AUC, calculated from IPGTT, were highly raised by about 109% and 55% in C-HF and P-HF groups as compared to C and P groups. Treatment of fructose rats with isolated pumpkin seed proteins exhibited low plasma glucose at all time points and decreased AUC by about 29% when compared to C-HF. Moreover, feeding P diet led to low AUC values and plasma glucose at 60 min than C diet in rats without metabolic syndrome induction.
Plasma glucose response and area under theglucose curve (glucose-AUC)to IPGTT in rats fed pumpkin protein or casein with or without high fructose diets for 60 days. Data are presented as mean ± SD and refer to 6 rats in each group. aP < 0.05, pumpkin protein vs. casein with or without fructose (P vs. C, P-HF vs. C-HF); bP < 0.05, fructose treatment vs. no fructose treatment (C-HF vs. C, P-HF vs. P)
Metabolic and hormonal data
Plasma D-glucose concentrations were significantly increased by about 26% and 24% in C-HF and P-HF groups relative to C and P groups, respectively (Table 5) after 2 months of experiment. Feeding pumpkin seed proteins with or without fructose decreased by about 7% and 8% glucose levels. Insulin concentrations were 112% and 124% greater in C-HF and P-HF-fed rats as compared to C and P rats. Furthermore, pumpkin seed proteins added reduced by 7% and 12% plasma insulin in P-HF and P, respectively, as compared to C-HF and C. The insulinogenic index was raised by 22% and 24% in C-HF and P-HF rats as compared to C and P rats. Interestingly, addition of pumpkin seed proteins to P and P-HF diets enhanced by 10% and 12% IGI compared to C and C-HF diets. HOMA-IR values were elevated in animals fed fructose. The exposure to the pumpkin diet exerted a decrease in the HOMA-IR in both P and P-HF groups. The results of HOMA-β showed an increase by about 32% and 38% in C-HF and P-HF as compared to C and P, respectively and by 9% in P-HF than in C-HF. Plasma fructose levels raised significantly (P < 0.05) in HF rats but treatment with pumpkin seed proteins reduced these values.
Effects on WAT and muscle tissues lipid and protein oxidation
The results shown in Table 6 demonstrated higher levels of WAT and muscle tissues TBARS in high fructose fed rats compared to controls. In addition, TBARS content was significantly reduced in WAT from rats fed pumpkin seed proteins with or without fructose. LHP concentrations were significantly higher in rats exposed to fructose in both muscle and WAT. The consumption of pumpkin seed proteins reduced the WAT LHP in P and P-HF groups without modifying those of the muscle. NO concentrations were significantly lower in both muscle and WAT of high fructose-fed animals, while, carbonyls were significantly higher. Compared to the C-HF-fed group, the P-HF fed rats exhibited high WAT carbonyl levels. Rats adapted to the P diet had significantly lowered carbonyls in muscle and WAT compared to C diet.
Effects on WAT and muscle tissue antioxidant enzyme activities
As depicted in Fig. 2, after an overnight starvation, the SOD activity was decreased in WAT and increased in muscle in C-HF when compared to C, while, the effect was inversed in P-HF compared to P. Moreover, after 2 months of experiment, treatment of fructose rats with pumpkin seeds protein caused high muscle and low WAT SOD activity relative to C-HF. In P group, an increase in SOD activity was observed in muscle and WAT than in C group. The results of CAT activity showed a significant elevation (P < 0.05) in muscle and reduction in WAT in C-HF than in C. When compared to P group, P-HF group showed a decrease in CAT muscle and an increase in WAT. Exposition of fructose rats to pumpkin seed proteins attenuated muscle CAT and enhanced it in WAT as compared to C-HF. Feeding P diet led to high muscle and low WAT CAT activity than C feeding. Ingestion of C-HF diet produced a significant increase in GSH-Px activity (P < 0.05) in muscle and WAT than C diet, whereas, a decrease was obtained when rats received P-HF diet than P diet. Supplementation of fructose diet with purified pumpkin seed proteins reduced significantly GSH-Px activity (P < 0.05) even in muscle and WAT than those reported in C-HF, while an increase was found in P compared to C.
Antioxidant enzymatic activities of adipose and muscle tissue in rats fed pumpkin protein or casein with or without high fructose diet for 60 days. Data are presented as mean ± SD and refer to 6 rats in each group. aP<0.05, pumpkin protein with and without fructose vs. casein with or without fructose (P vs. C, P-HF vs. C-HF); bP < 0.05, fructose treatment vs. no fructose treatment (C-HF vs. C, P-HF vs. P)
Effects on plasma cytokines
As shown in Table 7, after 2 months of feeding, plasma TNF-α concentrations were about 49% and 33% increase in C-HF and P-HF groups when compared to C and P groups, respectively. IL-6 levels were significantly higher by about 17% and 16% in C-HF and P-HF rats compared to controls, respectively. Treatment of HF rats with pumpkin seed proteins led to reduction in TNF-α by 12% as compared to C-HF without changing IL-6 contents. In addition, no significant changes were observed in P group compared to C group.
Discussion
Pumpkin is a plant that has been used frequently as functional food or medicine. Hence, this study explores the capacity of purified pumpkin seed proteins to prevent the development of metabolic syndrome induced by a high-fructose diet in rats.
The results from the current study revealed that despite virtually identical final body weight, BMI and food efficiency in both fructose-fed rats at the end of a 60-day period, the wet weight of the WAT was, as a rule, significantly greater in C-HF as compared to C. Similar results were observed by Shahraki et al. (2011). In other words, although C-HF feeding reduced food intake due probably to high amounts of energy production, the wet weight of the WAT was higher as compared to C diet. We also showed that C-HF feeding induced WAT hypertrophy, which agree with the results of Park et al. (2018) who showed cardiac hypertrophy in fructose-fed rats. Insulin is involved in the regulation of body adiposity via its actions in the central nervous system to inhibit food intake and increase energy expenditure. Rats adapted to the P-HF diet gained more weight, despite stabilization of food intake, absolute and relative white and epididymal adipose tissue and muscle weights than P diet, supposing low energy expenditure. Interestingly, our data showed that pumpkin seed proteins consumption prevented the adiposity index caused by the HF diet, despite equivalent body weight, weight gain, food intake when compared to C-HF diet, indicating that this beneficial effect may originate from reduced adiposity index.
Furthermore, these data imply that WAT weight gain following fructose ingestion may be responsible for impaired insulin sensitivity, HOMA-β, insulinogenic index, plasma D-glucose and insulin concentrations after the overnight starvation probably due to an alteration in the process of glucose recognition by the β cells as an insulinotropic agent or defects on glucose transporters and enzymes involved in glucose metabolism (El Mesallamy et al. 2010; Kannappan and Anuradha 2009). These experimental results are in agreement with previous studies (Dupas et al. 2017; Madani et al. 2012). Additionally, the HF diet is responsible for inducing physiological changes, such as tolerance to D-glucose and area under the curve. Thus, the plasma D-glucose level at 0, 15, 30, 60 and 120 min of the IPGTT, as well as the area under the curve (AUC) for such concentrations during the IPGTT were higher in chronic fructose feeding than in the controls. Such an alteration appeared mainly attributed to the impairment of insulin sensitivity, as indicated by the increase in HOMA-IR, HOMA-β and insulinogenic index. These findings are in close agreement with previous studies showing the diabetogenic effect of a high-fructose diet (Kalita et al. 2020; Tran et al. 2009; Yokozawa et al. 2008). However, rats assigned to the P-HF diet displayed an improvement of insulin resistance, glucose tolerance and decreased values for glucose AUC for IPGTT, a significant reduction in glucose, insulin, insulinogenic index, HOMA-β, and fructose levels compared to rats following the C-HF diet, suggesting that dietary proteins source may affect glucose metabolism differentially. Low glucose and high plasma insulin and number of β cells and lipid peroxidation in pancreas were observed in streptozotocin (STZ) diabetic rats-fed pumpkin, suggesting that pumpkin may play a role in both the renewal and the recovery of partially destroyed β cells in STZ diabetic rats (Xia and Wang 2007). Indeed, in our investigation, these favorable changes occurred without any loss of body weight but related to improvement of adiposity index, suggest that pumpkin seed proteins are protected against the development of glucose intolerance and insulin resistance induced by chronic fructose ingestion. Taken together, these data indicate that inclusion of pumpkin seed proteins to fructose-treated rats might be a safe strategy for treatment of the metabolic syndrome. Therefore, the plausible mechanism by which pumpkin proteins prevented insulin resistance is not related to the increased pancreatic secretion of insulin from beta cells of islets of Langerhans, as the values are lower than in C-HF, but may have at least its origin in the enhanced peripheral utilization of glucose and/or increased insulin receptor binding. On the other hand, it is possible that the presence of high levels of arginine (12.4vs. 3.9 g/100 g) and cystine (3.1 vs. 0.4 g/100 g) in pumpkin proteins may contribute to the improvement of glycemic homeostasis and insulin resistance induced by chronic fructose, which could prevent the destruction of cells that synthesize insulin and even regenerate them. Gannon and Nutall (2002) demonstrated that arginine limited the rise in blood glucose without stimulating insulin secretion after oral glucose administration. Xia and Wang (2006) reported, in streptozotocin-induced diabetic rats fed pumpkin fruit extract, low blood glucose and HbA1c, and high plasma insulin and total haemoglobin. Studies have shown that a pumpkin-rich diet has pharmacological activity in lowering blood glucose (Cai et al. 2003; Quanhong et al. 2005; Xiong and Cao 2001).
In agreement with our previous reports (Madani et al. 2012, 2015), we observed that fructose-fed rats display oxidative stress in both WAT and skeletal muscle after 8 weeks of experiment reflected in the elevated TBARS and LHP with respect to those of control animals. This abnormal stimulation of lipid peroxidation is associated with changes in the antioxidant enzymatic systems such as GSH-Px, SOD and CAT activities that may be an adaptive response to conditions of higher peroxidative stress in these tissues and to reduced plasma ascorbic acid and glutathione. The hydrogen peroxide and organic peroxides inhibited SOD activity and the lowered SOD activity could favor a greater presence of superoxide anions in the muscle and WAT tissues. High fructose increases protein fructosylation and the formation of reactive oxygen species (ROS) (Al-Okbi et al. 2014; Lim et al. 2010). Furthermore, the protein carbonyl increased in both tissues is suggestive of oxidative damage in addition to chemical modification, indicating that the high tissues carbonyl radicals can be produced after the attack of the •OH radical against the residues of arginine, lysine and proline (Kayama et al. 2015; Radi 2018).
We also found that fructose enriched diet resulted in significant reduction in nitric oxide (NO) levels in both tissues and concur with those observed in patients with MetS (Simão et al. 2010) and in rats (Madani et al. 2015; Mellouk et al. 2012b), supposing that hyperglycemia, hyperinsulinemia and altered insulin resistance may be the cause of this effect. Furthermore, the low NO contents might be explained by a higher level of O2 −• which can rapidly bound to NO and produce nitric peroxide (ONOO−), oxidant molecule harmful to vascular function (Zweier et al. 2011). Our data provide evidence that regardless of the type of protein intake, TBARS and LHP levels in both tissues were significantly declined in fructose groups-fed pumpkin seed proteins when compared to those fed casein, showing that improving oxidative lipid damage may be explained, at least by amelioration of insulin resistance. Moreover, we noticed that in the WAT tissue, SOD and GSH-Px levels were depleted, while CAT was raised. However, in the gastrocnemius muscle, GPx and CAT levels were lower and SOD was higher. Therefore, it can be supposed that reduced accumulation of H2O2 in the WAT tissue as result of increased detoxification by the CAT in rats fed the PHF diet might lead to the low glutathione levels inhibiting the GSHPx mediated reduction of H2O2 and organic hydroperoxides. In addition, depletion WAT SOD and GSH-Px activities in P-HF when compared to C-HF, may result from lower quantities of O2 −• and H2O2 present in tissues. While, in muscle, SOD was significantly higher and H2O2 is detoxified either by the CAT and GSH-Px. It is also interesting to note that pumpkin proteins in rats without metabolic syndrome reduced WAT TBARS and LHP and WAT and muscle carbonyls in comparison to C, increasing SOD and GSH-Px activities in both tissues and raising CAT in muscle and lowering it in WAT. On the other hand, the antioxidant protective effect of pumpkin proteins might be related to the presence of non-proteinous components in these proteins that could be responsible on the oxidant-lowering effect.
In our study, 8 weeks of fructose supplementation was sufficient to significantly increase plasma TNF-α and IL-6 levels compared to those reported in rats without metabolic syndrome induction. Hussein et al. (2014) found high serum TNF-α and IL-6 levels in rats fed 60% fructose for 60 days. The TNF-α level was reported to be markedly elevated in fructose-induced metabolic syndrome rats (Senaphan et al. 2015). The raise in TNF-α and IL-6 in fructose-exposed rats could be associated to NF-kB activation which leads to a rise in ROS levels and oxidative damage. NF-kB activation increased the expression of pro-inflammatory cytokines and stimulation of inflammatory cascade in high fructose diet (Kuhad et al. 2009). These experimental data may suppose that insulin resistance noticed in HF animals may be responsible for the higher production of cytokines as consequence of reduced anti-inflammatory effect of insulin in insulin-resistant states (Fernandez-Real and Ricart 2003). Alternatively, increased adiposity causes high inflammation and fructose intake leads to elevated free fatty acids which further leads to high inflammation (Tripathy et al. 2003). Exposure of adipose tissue to oxidative stress determines the development of a systemic inflammation state (Otani 2011). These results are supported by previous reports in human metabolic syndrome (Lim et al. 2010) and in high-fructose rats (Al-Okbi et al. 2014; Madani et al. 2012; Tian et al. 2013). Following pumpkin proteins ingestion in fructose rats reversed the altered status of inflammatory markers and led to the reduction of plasma TNF-α without affecting IL-6 levels compared to those of C-HF. Therefore, it can be expected that the improvement in TNF-α levels may be linked to an improvement in insulin resistance and a decrease in the adiposity index during pumpkin proteins treatment that further leads to a reduction in inflammation. Moreover, the presence of anti-inflammatory molecules such as arginine, in pumpkin proteins, may partly explain their protective action against inflammation induced by fructose-enriched diet. Arginine is suggested to have anti-inflammatory properties, by reducing lipotoxicity and/or affecting postprandial metabolism. Arginine is highly available in pumpkin proteins, thus beneficial effects of pumpkin protein could be partially driven by arginine. Studies have shown that pumpkin has antidiabetic (Adams et al. 2011; Boaduo et al. 2014), antioxidant and anti-inflammatory effects (Nawirska-Olszańska et al. 2013).
Conclusions
In this study we highlighted that the treatment with pumpkin seeds protein counteracted the fructose alterations by improving glucose, insulin, insulinogenic index, HOMA-IR, HOMA-β, glucose tolerance and AUC, suggesting a protective role on complications associated with metabolic syndrome. In addition, they exert strong antioxidant and anti-inflammatory effects in fructose-induced metabolic syndrome rats. Further studies are needed to isolate and characterize bioactive molecules such as amino acids or peptides to demonstrate the pumpkin protein mechanism of action.
Abbreviations
- ANOVA:
-
Analysis of Variance
- AUC:
-
Area Under Curve
- BMI:
-
Body Mass Index
- BW:
-
Body Weight
- CAT:
-
Catalase
- EAT:
-
Epididymal Adipose Tissue
- Fru:
-
Fructose
- GSH-Px:
-
Glutathione Peroxidase
- HOMA-IR:
-
Homeostatic Model Assessment of Insulin Resistance
- IGI:
-
Insulinogenic Index
- IL-6:
-
Interleukin-6
- IPGTT:
-
Intraperitoneal Glucose Tolerance Test
- LHP:
-
Lipid Tissue Hydroperoxide
- MetS:
-
Metabolic Syndrome
- NO:
-
Nitric Oxide
- Prot:
-
Protein
- ROS:
-
Reactive Oxygen Species
- SD:
-
Standard Deviation
- SOD:
-
Superoxide Dismutase
- STZ:
-
Streptozotocin
- TBARS:
-
Thiobarbituric Acid-Reactive Substances
- TNF-α:
-
Tumor Necrosis Factor-α
- WAT:
-
White Adipose Tissue
References
Adams GG, Imran S, Wang S, Mohammad A, Kok S, Gray DA, Channell GA, Morrins GA, Harding SE (2011) The hypoglycaemic effect of pumpkins as anti-diabetic and functional medicines. Int Food Res J44:862–867. https://doi.org/10.1016/j.foodres.2011.03.016
Aebi H (1974) Catalase. In:Bergmeyer HU(ed)Methods of Enzymatic Analysis.Weinheim/NewYork, pp.673–684.https://doi.org/10.1016/b978-0-12-091302-2.50032-3
Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart CJC, James WPT, Loria CM, Smith JrS (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation120: 1640–1645. https://doi.org/10.1161/CIRCULATIONAHA.109.192644
Al-Okbi SY, Mohamed DA, Hamed TE, Esmail RSH (2014) Rice bran oil and pumpkin seed oil alleviate oxidative injury and fatty liver in rats fed high fructose diet.Pol. J Food Nutr Sci 64:127–133. https://doi.org/10.2478/pjfns-2013-0002
Badr SE, Shaaban M, Elkholy YM, Helal MH, Hamza AS, Masoud MS, El Safty MM (2011) Chemical composition and biological activity of ripe pumpkin fruits (Cucurbita pepo L.) cultivated in Egyptian habitats. Nat Prod Res 25:1524–1539. https://doi.org/10.1080/14786410903312991
Boaduo NK, Katerere D, Eloff JN, Naidoo V (2014) Evaluation of six plant species used traditionally in the treatment and control of diabetes mellitus in South Africa using in vitro methods. Pharm Biol 52:756–761. https://doi.org/10.3109/13880209.2013.869828
Cai T, Li Q, Yan H, Li N (2003) Study on the hypoglycemic action of pumpkin seed protein. J Chin Inst Food Sciand Technol 3:7–11
Caili F, Huan S, Quanhong L (2006) A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods Hum Nutr 61:73–80. https://doi.org/10.1007/s11130-006-0016-6
Cortas NK, Wakid NW (1990) Determination of inorganic nitrate in serum and urine by a kinetic cadmium reduction method. Clin Chem 36:1440–1443 PMID: 2387039
Council of European Communities (1987) Council instructions about the protection of living animals used in scientific investigations. Official Journal of the European Communities(OJEC) L358 (18.12.1986). Corrigendum OJEC: L117, 0505
Dotto JM, Chacha JS (2020) The potential of pumpkin seeds as a functional food ingredient. Sci. https://doi.org/10.1016/j.sciaf.2020.e00575. Afr10 e00575
Dupas J, Feray A, Goanvec C, Guernec A, Samson N, Bougaran P, Guerrero F, Mansourati J (2017) Metabolic syndrome and hypertension resulting from fructose enriched diet in Wistar rats. BioMed Res Int2017:2494067. https://doi.org/10.1155/2017/2494067
El Mesallamy HO, El Demerdash E, Hammad L, El Magdoub HM (2010) Effect of taurine supplementation on hyperhomocysteinemia and markers of oxidative stress in high fructose diet diet induced insulin resistance. Diabetol Metab Syndr 2:46. https://doi.org/10.1186/1758-5996-2-46
Eymand S, Genot C (2003) A modified xylenol orange method to evaluate formation of lipid hydroperoxides during storage and processing of small pelagic fish. Eur J Lipid Sci Technol 105:497–501. https://doi.org/10.1002/ejlt.200300768
Fernandez-Real JM, Ricart W (2003) Insulin resistanceand chronic cardiovascular inflammatory syndrome. Endocr Rev 24:278–301. https://doi.org/10.1210/er.2002-0010
Fokou E, Achu MB, Tchounguep FM (2004) Preliminary Nutritional Evaluation of Five Species of Egusi Seeds in Cameroon. Afr J Food Agric Nutr Dev 4:1–11. DOI:https://doi.org/10.4314/ajfand.v4i1.19151
Gannon MC, Nuttall JA, Nuttall FQ (2002) Oral arginine does not stimulate an increase in insulin concentration but delays glucose disposal. Am J Clin Nutr 76:1016–1022. https://doi.org/10.1093/ajcn/76.5.1016
Hussein SA, Abd El Hamid OM, Hemdan HS (2014) Protective effect of L-carnitine on metabolic disorders, oxidative stress, antioxidant status and inflammation in rat model of insulin resistante. Int J Biol Chem 8:21–36. https://doi.org/10.3923/ijbc.2014.21.36
Kalita H, Hazarika A, Devi R (2020) Withdrawal of high carbohydrate, high-fat diet alters status of trace elements to ameliorate metabolic syndrome in rats with type 2 diabetes mellitus. Can J Diabetes 44:317–326e1. https://doi.org/10.1016/j.jcjd.2019.10.001
Kannappan S, Anuradha CV (2009) Insulin sensitizing actions of fenugreek seed polyphenols, quercetin & metformin in a rat model and metformin in rat model. Indian J Med Res 129:401–408 PMID:19535835
Kayama Y, Raaz U, Jagger A, Adam M, Schellinger IN, Sakamoto M, Suzuki H, Toyama K, Spin JM, Tsao PS (2015) Diabetic cardiovascular disease induced by oxidative stress. Int J Mol Med 16:25234–25263. https://doi.org/10.3390/ijms161025234
Kim MY, Kim EJ, Kim YN, Choi C, Lee BH (2012) Comparison of the chemical compositions and nutritive values of various pumpkin (Cucurbitaceae) species and parts. Nutr Res Pract 6:21–27. https://doi.org/10.4162/nrp.2012.6.1.21
Kuhad AM, Bishnoi VT, Chopra K (2009) Suppression of NF-KB signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol Biochem Behav 92:251–259. https://doi.org/10.1016/j.pbb.2008.12.012
Levine RL, Garland D, Oliver CN, Amici A, Clement I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478. https://doi.org/10.1016/0076-6879(90)86141-h
Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH (2010) The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol 7:251–264. https://doi.org/10.1038/nrgastro.2010.41
Lim S, Taskinen MR, Boren J (2019) Crosstalk between nonalcoholic fatty liver disease and cardiometabolic syndrome. Obes Rev 20:599–611. https://doi.org/10.1111/obr.12820
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:256–275 PMID:14907713
Madani Z, Louchami K, Sener A, Malaisse WJ, Ait Yahia D (2012) Dietary sardine protein lowers insulin resistance, leptin and TNF-α and beneficially affects adipose tissue oxidative stress in rats with fructose-induced metabolic syndrome. Int JMol Med 29:311–318. https://doi.org/10.3892/ijmm.2011.836
Madani Z, Sener A, Malaisse WJ, Ait Yahia D (2015) Sardine protein diet increases plasma glucagon-likepeptide-1 levels and prevents tissue oxidative stress in rats fed a high-fructose diet. Mol Med Rep 12:7017–7026. https://doi.org/10.3892/mmr.2015.4324
Mellouk Z, Idrissi TH, Louchami K, Hupkens E, Malaisse WJ, Ait Yahia D, Sener A (2012a) The metabolic syndrome of fructose-fed rats effects of polyunsaturated 3 and 6 fatty acids. I. Intraperitoneal glucose tolerance test. Int J Mol Med 28:1087–1092. https://doi.org/10.3892/ijmm.2011.781
Mellouk Z, Sener A, Ait Yahia D, Malaisse WJ (2012b) The metabolic syndrome of fructose-fed rats: effects of polyunsaturated 3 and 6 fatty acids. VII. Oxidative stress. Mol Med Rep 6:1409–1412. https://doi.org/10.3892/mmr.2012.1093
Naderali EK, Williams G (2003) Prolonged endothelial-dependent and -independent arterial dysfunction induced in the rat by short-term feeding with a high-fat, high-sucrose diet, vol 166. Atherosclerosis, pp 253–259. https://doi.org/10.1016/S0021-9150(02)00367-2
Nawirska-Olszańska A, Kita A, Biesiada A, Sokół-Łętowska A, Kucharska AZ (2013) Characteristics of antioxidant activity and composition of pumpkin seed oils in 12 cultivars. Food Chem 139:155–161. https://doi.org/10.1016/j.foodchem.2013.02.009
Otani H (2011) Oxidative stress as pathogenesis of cardiovascular risk associated with metabolic syndrome. Antiox Redox. https://doi.org/10.1089/ars.2010.3739. Signal15:1911–1926
Park JH, Ku HJ, Kim JK, Park JW, Lee JH (2018) Amelioration of high fructose-induced cardiac hypertrophy by naringin. Sci Rep 8:9464–9474. https://doi.org/10.1038/s41598-018-27788-1
Quanhong L, Caili F, Yukui R, Guanghui H, Tongyi C (2005) Effects of protein-bound polysaccharide isolated from pumpkin on insulin in diabetic rats. Plant Foods Hum Nutr 60:13–16. doi: https://doi.org/10.1007/s11130-005-2536-x
Quintanilha AT, Packer L, Szyszio DJM, Racanelly TL, Davies KJA (1982) Membrane effects of vitamin E deficiency bioenergetic and surface charge density studies of skeletal muscle and liver mitochondria. Ann N Y Acad Sci 393:32–47. doi: https://doi.org/10.1111/j.1749-6632.1982.tb31230.x
Radi R (2018) Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. PNAS USA 115:5839–5848. https://doi.org/10.1073/pnas.1804932115
Santos RD, Valenti L, Romeo S (2019) Does nonalcoholic fatty liver disease cause cardiovascular disease? Current knowledge and gaps, vol 282. Atherosclerosis, pp 110–120. https://doi.org/10.1016/j.atherosclerosis.2019.01.029
Senaphan K, Kukongviriyapan U, Sangartit W, Pakdeechote P, Pannangpetch P, Prachaney P, Greenwald SE, KukongviriyapanV (2015) Ferulic acid alleviates changes in a rat model of metabolic syndrome induced by high-carbohydrate. High-Fat Diet Nutrients 7:6446–6464. https://doi.org/10.3390/nu7085283
Shahraki MR, Harati M, Shahraki AR (2011) Prevention of high fructose-induced metabolic syndrome in male wistar rats by aqueous Extract of Tamarindus Indicaseed. Acta Med Iran49:277–283.PMID: 21713743
Simão ANC, Lozovoy MAB, Simão TNC, Dichi JB, Matsuo T, Dichi I (2010) Nitric oxide enhancement and blood pressure decrease in patients with metabolic syndrome using soy protein or fish oil. Arq Bras Endocrinol Metabol 54:540545. https://doi.org/10.1590/s0004-27302010000600005
Stahl EP, Dhindsa DS, Lee SK, Sandesara PB, Chalasani NP, Sperling LS (2019) Nonalcoholic Fatty Liver Disease and the Heart: JACC State-of-the-Art Review. J Am Coll Cardiol 73:948–963. https://doi.org/10.1016/j.jacc.2018.11.050
Tian YF, He CT, Chen YT, Hsieh PS (2013) Lipoic acid suppresses portal endotoxemia-induced steatohepatitis and pancreatic inflammation in rats. World J Gastroenterol 19:2761–2771. https://doi.org/10.3748/wjg.v19.i18.2761
Tran LT, Yuen VG, McNeill JH (2009) The fructose-fed rat: a review on the mechanisms of fructose induced-insulin resistance and hypertension. Mol Cell Biochem 332:145–159. https://doi.org/10.1007/s11010-009-0184-4
Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, Dandona P (2003) Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects, vol 52. Diabetes, pp 2882–2887. https://doi.org/10.2337/diabetes.52.12.2882
Xia T, Wang Q (2006) Antihyperglycemic effect of Cucurbita ficifolia fruit extract in streptozotocin-induced diabetic rats. Fitoterapia 77:530–533. https://doi.org/10.1016/j.fitote.2006.06.008
Xia T, Wang Q (2007) Hypoglycaemic role of Cucurbita ficifolia (Cucurbitaceae) fruit extract in streptozotocin-induced diabetic rats. J Sci Food Agric 87:1753–1757. https://doi.org/10.1002/jsfa.2916
Xiong X, Cao J (2001) Study of extraction and isolation of effective pumpkin polysaccharide component and its reducing glycemia function. Chin J Mod Appl Pharm 18:662–664
Yokozawa T, Kim HJ, Cho EJ (2008) Gravinol ameliorates high-fructose-induced metabolicsyndrome through regulation of lipid metabolism and proinflammatory state in rats. J Agric Food Chem 56:5026–5032. https://doi.org/10.1021/jf800213f
Zweier JL, Chen CA, Druhan LJ (2011) S-glutathionylation reshapes our understanding of endothelial nitric oxide synthase uncoupling and nitric oxide/reactive oxygen species-mediated signaling. Antiox Redox Signal 14:1769–1775. https://doi.org/10.1089/ars.2011.3904
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Chenni Abdelkader: Investigation, Software, Formal analysis. Hamza Cherif Fatima Zohra: Methodology, Investigation. Erdogan Elius EA: Investigation. Daniela Lucchesi: Writing - Review & Editing. Laura Pucci: Writing - Review & Editing, Visualization. Ait Yahia Dalila: Conceptualization, Resources, Funding acquisition, Supervision and Writing - Original Draft.
Corresponding author
Ethics declarations
Competing interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelkader, C., Cherif, F.Z.H., Elius, E.A.E. et al. Pumpkin seed proteins (Cucurbita pepo L.) protect against diet-induced metabolic syndrome by improving insulin resistance and markers of oxidative stress and inflammation in rats. Biologia 77, 2677–2687 (2022). https://doi.org/10.1007/s11756-022-01112-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-022-01112-9