Abstract
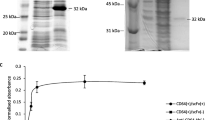
Denileukin diftitox is the first Food and Drug Administration (FDA) approved recombinant immunotoxin that has been prescribed in the treatment of some leukemia and lymphoma such as cutaneous T-cell lymphoma, chronic lymphocytic leukemia, and B-cell non-Hodgkin’s lymphoma in which the IL-2 receptor is overexpressed. This smart and directed toxin was named DT389IL-2 because it contains truncated diphtheria toxin (DT387), which is fused to human interleukin 2 (IL2). Today, since this protein is not stable for a long time, its production is dependent on customer demand. Aggregation is an important obstacle in the recombinant production of heterologous proteins, especially those with therapeutic properties. This study aimed to synthesize denileukin diftitox in the presence of Artemin as an efficient chaperone in the E. coli expression system, as well as to assess the soluble/aggregation of the synthesized protein. Two plasmids, one containing the denileukin diftitox protein-coding gene and the other carrying artemin gene were simultaneously transferred into E. coli BL21 (DE3). The transformed bacteria were then induced to overexpress the mentioned proteins. The soluble expression of the proteins was confirmed by the native PAGE technique. The results confirmed that the soluble expression of the denileukin diftitox protein significantly increased in the presence of Artemin as a new chaperon protein. To conclude, this strategy can be utilized for the soluble expression and production of this protein as a smart and directed toxin for the treatment of recurrent cutaneous T-cell lymphoma (CTCL).





Similar content being viewed by others
Data availability
These are available.
Abbreviations
- FDA:
-
U.S. Food and Drug Administration
- IL2:
-
Interleukin 2
- CTCL:
-
Cutaneous T-Cell Lymphoma
- DT:
-
Diphtheria Toxin
- HD:
-
Hodgkin’s Disease
- ATL:
-
Adult T-Cell Leukemia
- HSP60:
-
Heat Shock Proteins
- IPTG:
-
Isopropyl-ß-thiogalactopyranoside
- EDTA:
-
Ethylenediaminetetraacetic acid
- SDS:
-
Sodium Dodecyl Sulfate
- PMSF:
-
Phenylmethylsulfonyl Fluoride
- PAGE:
-
Polyacrylamide Gel Electrophoresis
- APS:
-
Ammonium Persulfate
References
Baneyx F, Mujacic M (2004) Recombinant protein folding and misfolding in Escherichia coli. Nat Biotechnol 22(11):1399–1408. https://doi.org/10.1038/nbt1029
Bayat S, Zeinoddini M, Azizi A, Khalili MN (2019) Co-Solvents effects on the stability of recombinant immunotoxin denileukin diftitox: Structure and function assessment. Iranian J Sci Technol Trans A Sci 43:2091–2097. https://doi.org/10.1007/s40995-019-00676-7
Chen T, Amons R, Clegg JS, Warner AH, MacRae TH (2003) Molecular characterization of Artemin and ferritin from Artemia franciscana. Eur J Biochem 270(1):137–145. https://doi.org/10.1046/j.1432-1033.2003.03373.x
Chen T, Villeneuve TS, Garant KA, Amons R, MacRae TH (2007) Functional characterization of Artemin, a ferritin homolog synthesized in Artemia embryos during encystment and diapause. FEBS J 274(4):1093–1101. https://doi.org/10.1111/j.1742-4658.2007.05659.x
Cheung LS, Fu J, Kumar P, Kumar A, Urbanowski ME, Ihms EA et al (2019) Second-generation IL-2 receptor-tarheted diphtheria fusion toxin exhibits antitumor activity and synergy with anti-PD-1 in melanoma. PNAS 116(8):3100–3105
De Herdt E, Slegers H, Kondo M (1979) Identification and characterization of a 19 S complex containing a 27000 Mr protein in Artemia salina Eur J Biochem 96(3):423–430. https://doi.org/10.1111/j.1432-1033.1979.tb13054.x
De Marco A (2007) Protocol for preparing proteins with improved solubility by co-expressing with molecular chaperones in Escherichia coli Nat Protoc 2(10):2632–2639. https://doi.org/10.1038/nprot.2007.400
Deuerling E, Schulze-Specking A, Tomoyasu T, Mogk A, Bukau B (1999) Trigger factor and DnaK cooperate in folding of newly synthesized proteins. Nature 400(6745):693–696. https://doi.org/10.1038/23301
Dobson CM (2003) Protein folding and misfolding. Nature 426(6968):884–890. https://doi.org/10.1038/nature02261
Dobson CM (2004) Principles of protein folding, misfolding and aggregation. Semin Cell Dev Biol 15(1):3–16. https://doi.org/10.1016/j.semcdb.2003.12.008
Foss FM (2000) DAB389IL-2 (denileukin diftitox, ONTAK): a new fusion protein technology. Clin Lymph 1:S27–S31. https://doi.org/10.3816/CLM.2000.s.005
Foss FM, Bacha P, Osann KE, Demierre MF, Bell T, Kuzel T (2001) Biological correlates of acute hypersensitivity events with DAB389IL-2 (denileukin diftitox, ONTAK®) in cutaneous T-cell lymphoma: Decreased frequency and severity with steroid premedication. Clin Lymph 1(4):298–302. https://doi.org/10.3816/CLM.2001.n.005
Galloway CA, Sowden MP, Smith HC (2003) Increasing the yield of soluble recombinant protein expressed in E. coli by induction during late log phase. Biotechniques 34(3):524–530. https://doi.org/10.2144/03343st04
Georgiou G, Valax P (1996) Expression of correctly folded proteins in Escherichia coli Curr Opin Biotechnol 7(2):190–197. https://doi.org/10.1016/s0958-1669(96)80012-7
Georgopoulos C, Welch WJ (1993) Role of the major heat shock proteins as molecular chaperones. Ann Rev Cell Biol 9(1):601–634. https://doi.org/10.1146/annurev.cb.09.110193
Ghodrati Siahmazgi M, Nasiri Khalili MA, Zeinoddini M, Ahmadpour F, Khodadadi S (2019) Purification and characterization of DT389GCSF fusion protein:A unique immunotoxin against the human granulocyte-colony stimulating factor receptor. Int J Peptide Res Ther 26(2):767–774. https://doi.org/10.1007/s10989-019-09884-6
Hendrick JP, Hartl FU (1993) Molecular chaperone functions of heat-shock proteins. Ann Rev Biochem 62(1):349–384. https://doi.org/10.1146/annurev.bi.62.070193.002025
Jolly C, Morimoto RI (2000) Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst 92(19):1564–1572. https://doi.org/10.1093/jnci/92.19.1564
Khosrowabadi E, Takalloo Z, Sajedi RH, Khajeh K (2018) Improving the soluble expression of aequorin in Escherichia coli using the chaperone-based approach by co-expression with Artemin. Prep Biochem Biotechnol 48(6):483–489. https://doi.org/10.1080/10826068.2018.1466152
Kumar MS, Kapoor M, Sinha S, Reddy GB (2005) Insights into Hydrophobicity and the Chaperone-like Function of αA-and αB-crystallins an isothermal titration calorimetric study. J Biol Chem 280(23):21726–21730. https://doi.org/10.1074/jbc.M500405200
Martinez-Alonso M, Garcia-Fruitos E, Ferrer-Miralles N, Rinas U, Villaverde A (2010) Side effects of chaperone gene co-expression in recombinant protein production. Microb Cell Fact 9:64. https://doi.org/10.1186/1475-2859-9-64
Moghaddas M, Zeinoddini M, Saeedinia AR, Bayat Sh (2018) Structure and functional assessment of diphtheria fusion toxin: DT389GCSF. J Bionanosci 12:240–44. https://doi.org/10.1166/jbns.2018.1517
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxcity assays. J Immunol Methods 65(1–2):55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Ohmachi K, Ando K, Ogura M, Uchida T, Tobinai K, Maruyama D, Namiki M, Nakanishi T (2018) E7777 in Jamanese patients with relapse/refravtory peripheral and cutaneous T-cell lymphoma: A phase I study. Cancer Sci 109:794–802. https://doi.org/10.1111/cas.13513
Ow DS, Lim DY, Nissom PM, Camattari A, Wong VV (2010) Co-expression of Skp and FkpA chaperones improves cell viability and alters the global expression of stress response genes during scFvD1. 3 production. Microb Cell Fact 9(1):22. https://doi.org/10.1186/1475-2859-9-22
Perentesis JP, Genbauffe FS, Veldman SA, Galeotti CL, Livingston DM, Bodley JW, Murphy JR (1988) Expression of diphtheria toxin fragment A and hormone-toxin fusion protein in toxin-resistant yeast mutants. Proc Natl Acad Sci USA 85:8386–8390. https://doi.org/10.1073/pnas.85.22.8386
Poon S, Treweek TM, Wilson MR, Easterbrook-Smith SB, Carver JA (2002) Clusterin is an extracellular chaperone that specifically interacts with slowly aggregating proteins on their off-folding pathway. FEBS Lett 513(2–3):259–266. https://doi.org/10.1016/s0014-5793(02)02326-8
Rasti B, Shahangian SS, Sajedi RH, Taghdir M, Hasannia S, Ranjbar B (2009) Sequence and structural analysis of Artemin based on ferritin: a comparative study. Biochim Biophy Acta 1794(10):1407–1413. https://doi.org/10.1016/j.bbapap.2009.05.005
Rodríguez V, Asenjo JA, Andrews BA (2014) Design and implementation of a high yield production system for recombinant expression of peptides. Microb Cell Fact 13(1):65. https://doi.org/10.1186/1475-2859-13-65
Sayadmanesh A, Ebrahimi F, Hajizade A, Rostamian M, Keshavarz H (2013) Expression and purification of neurotoxin-associated protein HA-33/A from Clostridium botulinum and evaluation of its antigenicity. Iranian Biomed J 17(4):165–170. https://doi.org/10.6091/ibj.1216.2013
Shahangian SS, Rasti B, Sajedi RH, Khodarahmi R, Taghdir M, Ranjbar B (2011) Artemin as an efficient molecular chaperone. Protein J 30(8):549–557. https://doi.org/10.1007/s10930-011-9359-4
Tiroli AO, Ramos CH (2007) Biochemical and biophysical characterization of small heat shock proteins from sugarcane: Involvement of a specific region located at the N-terminus with substrate specificity. Int J Biochem Cell Biol 39(4):818–831. https://doi.org/10.1016/j.biocel.2007.01.014
Turturro F (2007) Denileukin diftitox: a biotherapeutic paradigm shift in the treatment of lymphoid-derived disorders. Expert Rev Anticancer Ther 7(1):11–17. https://doi.org/10.1586/14737140.7.1.11
Williams DP, Snider CE, Strom TB, Murphy JR (1990) Structure/function analysis of interleukin-2-toxin (DAB486-IL-2). Fragment B sequences required for the delivery of fragment A to the cytosol of target cells. J Biol Chem 265:11885–11889
Woo JH, Liu YY, Mathias A, Stavrou S, Wang Z et al (2002) Gene optimization is necessary to express a biavalent anti-human anti-T cell immunotoxin in Pichia pastoris Protein Exp Pur 25:270–282
Zarkar N, Khalili MA, Ahmadpour F, Khodadadi S, Zeinoddini M (2019) In Silico and in Vitro Evaluation of Deamidation Effects on the Stability of the Fusion Toxin DAB389IL-2. Curr Proteom 16(4):307–313. https://doi.org/10.2174/1570164616666190131150033
Zarkar N, Khalili MA, Khodadadi S, Zeinoddini M, Ahmadpour F (2020) Expression and purification of soluble and functional fusion protein DAB389IL-2 into the E. coli strain Rosetta-gami (DE3). Biotechnol Appl Biochem 67(2):206–212. https://doi.org/10.1002/bab.1833
Acknowledgements
We would like to thank the research council of Malek Ashtar Universities of Technology (MUT) for the instrument support of this investigation.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design: Mehdi Zeinoddini, Ali Reza Saeeidinia, Reza Hasan Sajedi; experiments and data collection: Mohamad Najarasl; analysis and interpretation of results: Mehdi Zeinoddini, Ali Reza Saeeidinia, Mohamad Najarasl, Reza Hasan Sajedi; draft manuscript preparation: Mohamad Najarasl, Mehdi Zeinoddini. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethic approval and consent to participate
In this research, we did not use human or animal experiments.
Consent for publication
All authors agree for the article publication.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Najarasl, M., Zeinoddini, M., Saeeidinia, A.R. et al. The co-expression of denileukin diftitox immunotoxin with Artemin: soluble and aggregation analysis in presence of an efficient protein chaperone. Biologia 76, 3421–3428 (2021). https://doi.org/10.1007/s11756-021-00846-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11756-021-00846-2