Abstract
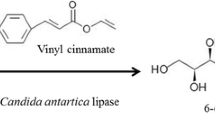
The esterification of some natural antioxidants such as cinnamic acid derivatives and ascorbic acid in non-aqueous media, catalyzed by immobilized lipases from Candida antarctica and Rhizomucor miehei, was investigated. The alcohol chain length affected the rate of esterification of cinnamic acids by both lipases. Higher reaction rates were observed when the esterification was carried out with medium- or long-chain alcohols. The rate also depended on aromatic acid structure. The reactivity of the carboxylic function of the cinnamic acids was affected by electron-donating substituents in the aromatic ring. Higher yields were observed for the esterification of p-hydroxyphenylacetic acid (97%) catalyzed by C. antarctica lipase and for the esterification of cinnamic acid (59%) catalyzed by R. miehei lipase. Candida antarctica lipase was more suitable for producing ascorbic acid fatty esters, catalyzing with a relatively high yield (up to 65% within 24 h) the regioselective esterification of ascorbic acid with various fatty acids in 2-methyl-2-propanol. The reaction rate and yield depended on the fatty acid chain length and on the molar ratio of reactants. All ascorbic acid fatty esters produced by this procedure exhibited a significant antioxidant activity in a micellar substrate composed of linoleic acid.
Similar content being viewed by others
References
Sherwin, E.R., Antioxidants for Food Fats and Oils, J. Am. Oil. Chem. Soc. 49:468–472 (1972).
Kochhar, S.P., and J.B. Rossell, Detection, Estimation and Evaluation of Antioxidants in Food Systems, in Food Antioxidants, edited by B.J.F. Hudson, Elsevier Science Publishers, London, 1990, pp. 19–22.
Pratt, D.E., and B.J.F. Hudson, Natural Antioxidants Not Exploited Commercially, in Food Antioxidants, edited by B.J.F. Hudson, Elsevier Science Publishers, London, 1990, p. 171.
Shahidi, F., P.K. Janitha, and P.D. Wanasundara, Phenolic Antioxidants, Crit. Rev. Food Sci. Nutr. 32:67–75 (1992).
Schuler, P., Natural Antioxidants Exploited Commercially, in Food Antioxidants, edited by B.J.F. Hudson, Elsevier Science Publishers, London, 1990, pp. 113–127.
Wang, X.Y., P.A. Seib, and K.S. Ra, l-Ascorbic Acid and Its 2-Phosphorylated Derivatives in Selected Foods: Vitamin C Fortification and Antioxidants Properties, J. Food Sci. 60:1296–1299 (1992).
Larson, R.A., The Antioxidants of Higher Plants, Phytochemistry 27:969–978 (1988).
Graf, E., Antioxidant Potential of Ferulic Acid, Free Radicals Biol. 13:435–448 (1992).
Yagi, K., and N. Ohishi, Action of Ferulic Acid and Its Derivatives as Antioxidants, J. Nutr. Sci. Vitaminol. 25:127–130 (1979).
Liu, X.-Y., F.-L. Guo, L.-M. Wu, and Z.-L. Liu, Remarkable Enhancement of Antioxidant Activity of Vitamin C in an Artificial Bilayer by Making It Lipo-Soluble, Chem. Phys. Lipids 83:39–43 (1996).
Marinova, E.M., and N.V. Yanishlieva, Effect of Lipid Unsaturation on the Antioxidative Activity of Some Phenolic Acids, J. Am. Oil Chem. Soc. 71:427–434 (1994).
Chimi, H., J. Cillard, P. Cillard, and M. Rahmani, Peroxyl and Hydroxyl Radical Scavenging Activity of Some Natural Phenolic Antioxidants, Ibid.:307–312 (1991).
Han, D., O.S. Yi, and H.K. Shin, Antioxidative Effect of Ascorbic Acid Solubilized in Oils via Reversed Micelles, J. Food Sci. 55:247–249 (1990).
Ito, S., C. Kobayashi, and S. Ikeda, Jpn. Kokai Tokkyo Koho JP 09065864 (1997).
Gutman, A.L., E. Shkolnik, and M. Shapira, A Convenient Method for Enzymatic Benzyl-Alkyl Transesterification Under Mild Neutral Conditions, Tetrahedron 48:8775–8780 (1992).
Ballesteros, A., U. Bornscheuer, A. Capewell, D. Combes, J.-S. Condoret, K. Koening, F.N. Kolisis, A. Marty, U. Menge, T. Scheper, H. Stamatis, and A. Xenakis, Enzymes in Non-Conventional Phases, Biocatal. Biotransform. 13:1–42 (1995).
John, V.T., and G. Abraham, Lipase Catalysis and Its Applications, in Biocatalysis for Industry, edited by J.S. Dodrick, Plenum Press, New York, 1991, pp. 193–218.
Guyot, B., B. Bosquette, M. Pina, and J. Graille, Esterification of Phenolic Acids from Green Coffee with an Immobilized Lipase from Candida antarctica in Solvent-Free Medium, Biotechnol. Lett. 19:529–532 (1997).
Buisman, G.J.H., C.T.W. van Helteren, G.F.H. Kramer, J.W. Veldsink, J.T.P. Derksen, and F.P. Cuperus, Enzymatic Esterification of Functionalized Phenols for the Synthesis of Lipophilic Derivatives, Ibid.:131–136 (1998).
Enomoto, K., T. Miyamori, A. Sakimae, and R. Numazawa, Eur. Pat. Appl. EP, 401,704 (1990).
Sakashita, K., S. Miyamoto, and A. Sakimae, Eur. Pat. Appl. EP, 514,694 (1992).
Humeau, C., M. Girardin, D. Coulon, and A. Miclo, Synthesis of 6-O-Palmitoyl l-Ascorbic Acid Catalyzed by Candida antarctica Lipase, Biotechnol. Lett. 17:1091–1094 (1998).
Borneman, W.S., R.D. Hartley, W.H. Morrison, D.E. Akin, and L.G. Ljungdahl, Feruloyl and p-Coumaroyl Esterase from Anaerobic Fungi in Relation to Plant Cell Wall Degradation, Appl. Microbiol. Biotechnol. 33:345–351 (1990).
Nishimura, T., T. Kometani, H. Takii, Y. Terada, and S. Okada, Glucosylation of Caffeic Acid with Bacillus subtilis X-23 a-Amylase and a Description of the Glucosides, J. Ferment. Bioeng. 80:18–23 (1995).
Gandhi, N.N., S.B. Sawant, J.B. Joshi, and D. Mukesh, Lipozyme Deactivation by Butanol and Temperature, Enzyme Microb. Technol. 17:373–380 (1995).
Karamajc, M., and R. Amarowicz, Inhibition of Pancreatic Lipase by Phenolic Acids—Examination in vitro, Z. Naturforsch. C. Biochem. Biophys. Biol. Virol. 51:903–905 (1996).
Stamatis, H., V. Sereti, and F.N. Kolisis, Studies on the Enzymatic Synthesis of Sugar Esters in Organic Medium and Supercritical Carbon Dioxide, Chem. Biochem. Eng. Q. 12:151–156 (1998).
Tsitsimpikou, C., H. Stamatis, V. Sereti, H. Daflos, and F.N. Kolisis, Acylation of Glucose Catalysed by Lipases in Supercritical Carbon Dioxide, J. Chem. Technol. Biotechnol.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Stamatis, H., Sereti, V. & Kolisis, F.N. Studies on the enzymatic synthesis of lipophilic derivatives of natural antioxidants. J Amer Oil Chem Soc 76, 1505–1510 (1999). https://doi.org/10.1007/s11746-999-0193-1
Issue Date:
DOI: https://doi.org/10.1007/s11746-999-0193-1