Abstract
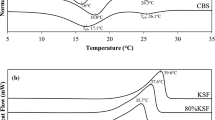
Two ternary systems of confectionery fats were studied. In the first system, lauric cocoa butter substitutes (CBS), anhydrous milk fat (AMF), and Malaysian cocoa butter (MCB) were blended. In the second system, high-melting fraction of milk fat (HMF42) was used to replace AMF and also was blended with CBS and MCB. CBS contained high concentrations of lauric (C12:0) and myristic (C14:0) acids, whereas palmitic (C16:0), stearic (C18:0), and oleic (C18:1) acid concentrations were higher in MCB. In addition, AMF and HMF42 contained appreciable amounts of short-chain fatty acids. CBS showed the highest melting enthalpy (143.1 J/g), followed by MCB (138.8 J/g), HMF42 (97.1 J/g), and AMF (72.9 J/g). The partial melting enthalpies at 20 and 30°C demonstrated formation of a eutectic along the binary blends of CBS/MCB, AMF/MCB, and HMF42/MCB. However, no eutectic effect was observed along the binary lines of AMF/CBS and HMF42/CBS. Characteristics of CBS included two strong spacings at 4.20 and 3.8 Å. MCB showed a strong spacing at 4.60 Å and a weak short-spacing at 4.20 Å. On the other hand, AMF exhibited a very weak short-spacing at 4.60 Å and two strong spacings at 4.20 and 3.8 Å, while HMF42 showed an intermediate short-spacing at 4.60 Å and also two strong short-spacings at 4.20 and 3.8 Å. Solid fat content (SFC) analyses at 20°C showed that CBS possessed the highest solid fat (91%), followed by MCB (82.4%), HMF42 (41.4%), and AMF (15.6%). However, at 30°C, MCB showed the highest SFC compared to the other fats. Results showed that a higher SFC in blends that contain HMF does not necessarily correlate with a stronger tendency to form the β polymorph.
Similar content being viewed by others
References
Paulicka, F.R., Phase Behavior of Fats in Confectionery Coatings, Proceedings of the Annual Production Conference 24:43–46 (1970).
Paulicka, F.R., Phase Behavior of Cocoa Butter Extender, Chem. Ind. (London) 17:835–839 (1973).
Lovegren, N.V., M.S. Gray, and R.O. Feuge, Polymorphic Changes in Mixtures of Confectionery Fats, J. Am. Oil Chem. Soc. 53:83–88 (1976).
Hogenbirk, G., Compatibility of Specialty Fats with Cocoa Butter, Manuf. Confect. 64:59–63 (1984).
Md. Ali, A.R., M.S. Embong, H. Omar, and C.H.Oh. Flingoh, Effect of Oleo-Disaturated Triacylglycerol Content on Properties of Palm Mid Fraction. Sal Stearin and Borneo Tallow Blends, J. Am. Oil Chem. Soc. 68:238–331 (1991).
Md. Ali, A.R., and C.H.Oh. Flingoh, Effect of Cocoa Butter on Compatibility of Specialty Fats, Elaeis 5:86–91 (1993).
Chapman, G.M., Cocoa Butter and Confectionery Fats. Studies Using Programmed Temperature X-Ray Diffraction and Differential Scanning Calroimetry, J. Am. Oil Chem. Soc. 48:824–830 (1971).
Timms, R.E., The Phase Behavior of Mixtures of Cocoa Butter and Milkfat, Lebensm. Wiss. Technol. 13:61–65 (1980).
Bigalli, G.L., R.D. Houseal, and D. Eichelberger, Practical Aspects of Eutectic Effect on Confectionery Fats and Their Mixtures, Manuf. Confect., 68:65–80 (1988).
Barna, C.M., R.W. Hartel, and S. Metin, Incorporation of Milkfat Fractions into Milk Chocolate, Proceedings of the Annual Production Conference 46:62–71 (1992).
Padley, F.B., Characterization of Cocoa Butter Equivalents and Their Determination in Chocolate, Paper presented at the General Assembly of the Technical Committee in Cambridge, June 1980.
Gordon, M.H., F.B. Padley, and R.E. Timms, Factors Influencing the Use of Vegetable Fats in Chocolate, Fette Seifen Anstrichm. 81:116–121 (1979).
Herzing, A.G., Eutectic Effects of Fats, Manuf. Confect. 69:83–87 (1989).
Md. Ali, A.R., and P.S. Dimick, Thermal Analysis of Palm Mid-Fraction, Cocoa Butter and Milkfat Blends by Differential Scanning Calorimetry, J. Am. Oil Chem. Soc. 71:803–806 (1994).
Md. Ali, A.R., M.S. Embong, and C.H.Oh. Flingoh, Interaction of Cocoa Butter Equivalent Component Fats in Ternary Blends, Elaeis 4:21–26 (1992).
Paquot, C., Solid Content Determination in Fats by NMR (low resolution nuclear magnetic resonance), IUPAC Standard Methods for the Analysis of Oils and Fats and Derivatives, Pergamon Press, New York, 1987, Method 2.150.
Timms, R.E., The Phase Behavior and Polymorphism of Milkfat, Milkfat Fractions and Fully Hardened Milkfat, Aust. J. Dairy Technol. 34:60–65 (1979).
Shukla, V.K.S., Cocoa Butter Properties and Quality, Lipid Technol. 5:54–57 (1995).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sabariah, S., Md. Ali, A.R. & Chong, C.L. Chemical and physical characteristics of cocoa butter substitutes, milk fat and malaysian cocoa butter blends. J Am Oil Chem Soc 75, 905–910 (1998). https://doi.org/10.1007/s11746-998-0265-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11746-998-0265-7