Abstract
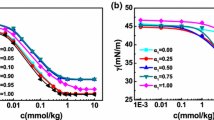
The effects of hydrophobic chain length on the interfacial and biological properties of diacyl d-glyceric acid (d-GA) sodium salts were evaluated based on interfacial tension analyses, dynamic light scattering (DLS), and antitrypsin activity. Of the four synthesized d-GA-derived surfactants [dihexanoyl d-GA sodium salt (diC6GA-Na), dioctanoyl d-GA sodium salt (diC8GA-Na), didecanoyl d-GA sodium salt (diC10GA-Na), and dilauroyl d-GA sodium salt (diC12GA-Na)], only those with C6, C8, and C10 acyl chains were investigated because diC12GA-Na were insoluble at room temperature. Together with our previous results, surface tensions at the critical micelle concentration (CMC) were 33.9 mN/m for diC6GA-Na, 25.5 mN/m for diC8GA-Na, and 27.9 mN/m for diC10GA-Na. Evaluation of assembly size via DLS and optical microscopy revealed that diC8GA-Na and diC10GA-Na formed large associates with average sizes ranging from 50 to 200 μm at concentrations 4–5 times greater than their CMC, whereas diC6GA-Na did not form such associates. In tryptic hydrolysis studies using N α-benzoyl-dl-arginine-4-nitroanilide as a substrate, diC8GA-Na exhibited an inhibitory effect on trypsin (trypsin specific activity: 0.26 ± 0.045 U/mg-protein) greater than that of diC10GA-Na (0.39 ± 0.10 U/mg-protein), whereas diC6GA-Na did not show antitrypsin activity. These results show that diC8GA-Na was the most bioactive of the evaluated diacyl d-glycerate surfactants.







Similar content being viewed by others
References
Serrano-Ruiz JC, Luque R, Sepúlveda-Escribano A (2011) Transformations of biomass-derived platform molecules: from high added-value chemicals to fuels via aqueous-phase processing. Chem Soc Rev 40:5266–5281
Kamm B, Kamm M (2004) Principles of biorefineries. Appl Microbiol Biotechnol 64:137–145
Demirbas MF, Balat M (2006) Recent advances on the production and utilization trends of bio-fuels: a global perspective. Energy Convers Manage 47:2371–2381
Claude S (1999) Research of new outlets for glycerol: recent developments in France. Fett/Lipid 101:101–104
Pagliaro M, Ciriminna R, Kimura H, Rossi M, Della Pina C (2007) From glycerol to value-added products. Angew Chem Int Ed 46:4434–4440
Zhou C-H, Beltramini JN, Fan Y-X, Lu GQ (2008) Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem Soc Rev 37:527–549
da Silva GP, Mack M, Contiero J (2009) Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv 27:30–39
Palmer JK (1956) Occurrence of d-glyceric acid in tobacco leaves. Science 123:415
Morrison RJ, Dekock PC (1959) Glyceric acid in broad bean (Vicia faba L.). Nature 184:819
Habe H, Fukuoka T, Kitamoto D, Sakaki K (2009) Biotransformation of glycerol to d-glyceric acid by Acetobacter tropicalis. Appl Microbiol Biotechnol 81:1033–1039
Habe H, Shimada Y, Yakushi T, Hattori H, Ano Y, Fukuoka T, Kitamoto D, Itagaki M, Watanabe K, Yanagishita H, Matsushita K, Sakaki K (2009) Microbial production of glyceric acid, an organic acid that can be mass produced from glycerol. Appl Environ Microbiol 75:7760–7766
Habe H, Shimada Y, Fukuoka T, Kitamoto D, Itagaki M, Watanabe K, Yanagishita H, Sakaki K (2009) Production of glyceric acid by Gluconobacter sp. NBRC3259 using raw glycerol. Biosci Biotechnol Biochem 73:1799–1805
Sato S, Morita N, Kitamoto D, Yakushi T, Matsushita K, Habe H (2013) Change in product selectivity in glyceric acid production from glycerol by Gluconobacter strains in the presence of methanol. AMB Expr 3(1):20
Handa SS, Sharma A, Chakraborti KK (1986) Natural products and plants as liver protecting drugs. Fitoterapia 57:307–351
Eriksson CJP, Saarenmaa TPS, Bykov IL, Heino PU (2007) Acceleration of ethanol and acetaldehyde oxidation by d-glycerate in rats. Metabolism 56:895–898
Habe H, Sato S, Fukuoka T, Kitamoto D, Sakaki K (2011) Effect of glyceric acid calcium salt on the viability of ethanol-dosed gastric cells. J Oleo Sci 60:327–331
Sato S, Kitamoto D, Habe H (2014) In vitro evaluation of glyceric acid and its glucosyl derivative, α-glucosylglyceric acid, as cell proliferation inducers and protective solutes. Biosci Biotechnol Biochem 78:1183–1186
Lesová K, Sturdíková M, Proksa B, Pigos M, Liptaj T (2001) OR-1: a mixture of esters of glyceric acid produced by Penicillium funiculosum and its antitrypsin activity. Folia Microbiol 46:21–23
Habe H, Fukuoka T, Sato S, Kitamoto D, Sakaki K (2011) Synthesis and evaluation of dioleoyl glyceric acids showing antitrypsin activity. J Oleo Sci 60:327–331
Sato S, Habe H, Fukuoka T, Kitamoto D, Sakaki K (2012) Stepwise synthesis of 2,3-O-dipalmitoyl-d-glyceric acid and an in vitro evaluation of its cytotoxicity. J Oleo Sci 61:337–341
Sato S, Habe H, Fukuoka T, Kitamoto D, Sakaki K (2011) Synthesis of dilinoleoyl-d-glyceric acid and evaluation of its cytotoxicity to human dermal fibroblast and endothelial cells. J Oleo Sci 60:483–487
Fukuoka T, Ikeda S, Habe H, Sato S, Sakai H, Abe M, Kitamoto D, Sakaki K (2012) Synthesis and interfacial properties of monoacyl glyceric acid as a new class of green surfactants. J Oleo Sci 61:343–348
Sato S, Nagata S, Imura T, Fukuoka T, Morita T, Takahashi Y, Kondo Y, Kitamoto D, Habe H (2016) Synthesis and characterization of dioctanoyl glycerate as water-soluble trypsin inhibitor. J Oleo Sci 65:251–256
Sato S, Nagata S, Kitamoto D, Takahashi Y, Kondo Y, Habe H (2017) Application of bio-based organic acid, d-glyceric acid: synthesis and interfacial property of dihexanoyl glycerate. J Environ Biotechnol 17:69–72
Rosen MJ (2004) Surfactants and interfacial phenomena, 3rd edn. John Wiley, New York
Kumar D, Rub MA (2017) Effect of anionic surfactant and temperature on micellization behavior of promethazine hydrochloride drug in absence and presence of urea. J Mol Liq 238:389–396
Azum N, Rub MA, Asiri AM (2016) Micellization and interfacial behavior of binary and ternary mixtures in aqueous medium. J Mol Liq 216:94–98
Rub MA, Khan F, Sheikh MS, Azum N, Asiri AM (2016) Tensiometric, fluorescence and 1H NMR study of mixed micellization of non-steroidal anti-inflammatory drug sodium salt of ibuprofen in the presence of non-ionic surfactant in aqueous/urea solutions. J Chem Thermodyn 96:196–207
Kumar D, Rub MA (2016) Aggregation behavior of amphiphilic drug promazine hydrochloride and sodium dodecylbenzenesulfonate mixtures under the influence of NaCl/urea at various concentration and temperatures. J Phys Org Chem 29:394–405
Kunieda H, Shinoda K (1976) Krafft points, critical micelle concentrations, surface tension, and solubilizing power of aqueous solutions of fluorinated surfactants. J Phys Chem 80:2468–2470
Pencer J, Hallett FR (2003) Effects of vesicle size and shape on static and dynamic light scattering measurements. Langmuir 19:7488–7497
Erlanger BF, Kokowsky N, Cohen W (1961) The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys 95:271–278
Kondo Y, Miyazawa H, Sakai H, Abe M, Yoshino N (2002) First anionic micelle with unusually long lifetime: self-assembly of fluorocarbon-hydrocarbon hybrid surfactant. J Am Chem Soc 124:6516–6517
Acknowledgements
This work was funded in part by KAKENHI No. 25850064.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Sato, S., Nagata, S., Kitamoto, D. et al. Comparative Study of Interfacial and Biological Properties in d-Glycerate-Derived Surfactants. J Am Oil Chem Soc 94, 1393–1401 (2017). https://doi.org/10.1007/s11746-017-3032-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-017-3032-9