Abstract
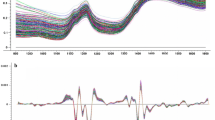
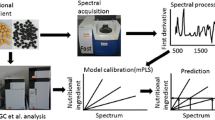
The mature seeds of soybean (Glycine max L. Merr) are a valuable source of high-quality edible lipids and protein. Despite dramatic breeding gains over the past 80 years, soybean oil continues to be oxidatively unstable, due to a high proportion of polyunsaturated triacylglycerols. Until recently, the majority of soybean oil underwent partial chemical hydrogenation. Mounting health concerns over trans fats, however, has increased breeding efforts to introgress mutant and biotechnological genetic alterations of soybean oil composition into high-yielding lines. As a result, there is an ongoing need to characterize fatty acid composition in a rapid, inexpensive and accurate manner. Gas chromatography is the most commonly used method, but near-infrared reflectance spectroscopy (NIRS) can be calibrated to non-destructively phenotype various seed compositions accurately and at a high throughput. Here we detail development of NIRS calibrations using intact seeds for every major soybean fatty acid breeding goal over an unprecedented range of oil composition. The NIRS calibrations were shown to be equivalent to destructive chemical analysis, and incorporation into a soybean phenotyping operation has the potential to dramatically reduce cost and accelerate phenotypic analysis.


Similar content being viewed by others
Abbreviations
- NIRS:
-
Near-infrared reflectance spectroscopy
- GC:
-
Gas chromatography
- MS:
-
Multiple scatter correction
- SEP:
-
Standard error of performance
- RMSEP:
-
Root mean square error of prediction
- RPD:
-
Relative prediction deviation
- FAs:
-
Fatty acid species
- PLS:
-
Partial least squares
References
Rotundo JL, Westgate ME (2009) Meta-analysis of environmental effects on soybean seed composition. Field Crop Res 110:147–156
Harwood JL (1980) 1—Plant acyl lipids: structure, distribution, and analysis A2. In: Stumpf PK (ed) Lipids: structure and function. Academic Press, New York, pp 1–55
Wilson RF (2004) Seed composition. In: Boerma HR, Specht J (eds) Soybeans: improvement, production, and uses. American Society of Agronomy, Madison, pp 621–677
Remig V, Franklin B, Margolis S, Kostas G, Nece T, Street JC (2010) Trans fats in America: a review of their use, consumption, health implications, and regulation. J Acad Nutr Diet. 110:585–592
Hunter JE, Zhang J, Kris-Etherton PM (2010) Cardiovascular disease risk of dietary stearic acid compared with trans, other saturated, and unsaturated fatty acids: a systematic review. Am J Clin Nutr 91:46–63
Yu S, Derr J, Etherton TD, Kris-Etherton PM (1995) Plasma cholesterol-predictive equations demonstrate that stearic acid is neutral and monounsaturated fatty acids are hypocholesterolemic. Am J Clin Nutr 61:1129–1139
Gillman JD, Bilyeu K (2012) Genes and alleles for quality traits on the soybean genetic/physical map. In: Willson RF (ed) Designing soybeans for 21st century markets. AOCS Press, Urbana, pp 67–96
Medic J, Atkinson C, Hurburgh CR (2014) Current knowledge in soybean composition. J Am Oil Chem Soc 91:363–384
Osborne BG (2006) Near-Infrared Spectroscopy in food analysis. Encyclopedia of analytical chemistry. Wiley, New York
Williams P (2007) Grains and seeds. In: Ozaki Y, McClure WF, Christy AA (eds) Near-infrared spectroscopy in food science and technology. Wiley, New York, pp 165–217
Hacisalihoglu G, Gustin JL, Louisma J, Armstrong P, Peter GF, Walker AR, Settles AM (2016) Enhanced single seed trait predictions in soybean (Glycine max) and robust calibration model transfer with near-infrared reflectance spectroscopy. J Agric Food Chem 64:1079–1086
Orman BA, Schumann RA (1991) Comparison of near-infrared spectroscopy calibration methods for the prediction of protein, oil, and starch in maize grain. J Agric Food Chem 39:883–886
Han S-I, Chae J-H, Bilyeu K, Shannon JG, Lee J-D (2014) Non-destructive determination of high oleic acid content in single soybean seeds by near infrared reflectance spectroscopy. J Am Oil Chem Soc 91:229–234
Pazdernik DL, Killam AS, Orf JH (1997) Analysis of amino and fatty acid composition in soybean seed, using near infrared reflectance spectroscopy. Agron J 89:679–685
Roberts CA, Ren C, Beuselinck PR, Benedict HR, Bilyeu K (2006) Fatty acid profiling of soybean cotyledons by near-infrared spectroscopy. Appl Spectrosc 60:1328–1333
Patil AG, Oak MD, Taware SP, Tamhankar SA, Rao VS (2010) Nondestructive estimation of fatty acid composition in soybean [Glycine max (L.) Merrill] seeds using near-infrared transmittance spectroscopy. Food Chem 120:1210–1217
Tillman BL, Gorbet DW, Person G (2006) Predicting oleic and linoleic acid content of single peanut seeds using near-infrared reflectance spectroscopy. Crop Sci 46:2121–2126
Hammond EG, Fehr WR (1983) Registration of A6 germplasm line of soybean. Crop Sci 23:192–193
Gillman JD, Stacey MG, Cui Y, Berg HR, Stacey G (2014) Deletions of the SACPD-C locus elevate seed stearic acid levels but also result in fatty acid and morphological alterations in nitrogen fixing nodules. BMC Plant Biol 14:143
Cantor SL, Hoag SW, Ellison CD, Khan MA, Lyon RC (2011) NIR Spectroscopy applications in the development of a compacted multiparticulate system for modified release. AAPS PharmSciTech 12:262–278
Martens M, Martens H (1986) Partial least squares regression. Elsevier Applied Science, London
Brown SD (1995) The Unscrambler®, Version 5.5. Multivariate analysis software for MS-DOS. J Chemom 9:527–529
Geladi P, MacDougall D, Martens H (1985) Linearization and scatter-correction for near-infrared reflectance spectra of meat. Appl Spectrosc 39:491–500
Spielbauer G, Armstrong P, Baier JW, Allen WB, Richardson K, Shen B, Settles AM (2009) High-throughput near-infrared reflectance spectroscopy for predicting quantitative and qualitative composition phenotypes of individual maize kernels. Cereal Chem 86:556–564
Baye TM, Pearson TC, Settles AM (2006) Development of a calibration to predict maize seed composition using single kernel near infrared spectroscopy. J Cereal Sci 43:236–243
Martens H, Naes T (1989) Assessment, validation and choice of calibration method. Wiley, New York
Williams PC, Norris KH (2001) Near-infrared technology in the agricultural and food industries. American Association of Cereal Chemists, Saint Paul
Igne B, Rippke GR, Hurburgh CR (2008) Measurement of whole soybean fatty acids by Near Infrared Spectroscopy. J Am Oil Chem Soc 85:1105–1113
Igne B, Roger J-M, Roussel S, Bellon-Maurel V, Hurburgh CR (2009) Improving the transfer of near infrared prediction models by orthogonal methods. Chemometr Intel Lab 99:57–65
Porep JU, Kammerer DR, Carle R (2015) On-line application of near infrared (NIR) spectroscopy in food production. Trends Food Sci Tech. 46:211–230
Acknowledgements
We acknowledge the excellent technical assistance of two USDA-ARS technicians Alexandria Berghaus and Jeremy Mullis who were primarily responsible for all field work and gas chromatography in support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no pertinent conflict of interest related to this study, which was partially supported by a grant from United Soybean Board (Project No. 1420-632-6605), and by USDA-Agricultural Research Service internal funding. Mention of any trademarks, vendors, or proprietary products does not constitute a guarantee or warranty of the product by the USDA and does not imply its approval to the exclusion of other products or vendors that may also be suitable. The USDA, Agricultural Research Service, Midwest Area, is an equal opportunity, affirmative action employer and all agency services are available without discrimination.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Karn, A., Heim, C., Flint-Garcia, S. et al. Development of Rigorous Fatty Acid Near-Infrared Spectroscopy Quantitation Methods in Support of Soybean Oil Improvement. J Am Oil Chem Soc 94, 69–76 (2017). https://doi.org/10.1007/s11746-016-2916-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-016-2916-4