Abstract
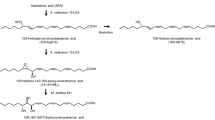
Arachidonyl alcohol rarely occurs in natural oils. It can be used as a substrate for production of several ether lipids possessing beneficial functions. Although arachidonyl alcohol has been produced on a laboratory scale by the chemical reduction of arachidonic acid, it will be difficult to scale up this process for industrial application. The aim of this study was to develop a new bioprocess for converting arachidonic acid to arachidonyl alcohol. Screening was conducted using 11 wax ester- (esters of fatty acids and fatty alcohols) producing strains reported in our previous study, and a single-cell oil containing arachidonic acid. A new strain, Acinetobacter species N-476-2, most effectively converted arachidonic acid to arachidonyl alcohol, which accumulated inside the cells as a wax ester. GC–MS, FT–IR, and NMR analyses showed that this strain reduced the carboxyl group of 5-cis,8-cis,11-cis,14-cis-arachidonic acid to a hydroxyl group without altering the position or configuration of the double bonds; the product was identified as 5-cis,8-cis,11-cis,14-cis-arachidonyl alcohol. A time-course study of cultivation showed that the amount of arachidonyl alcohol produced by the strain after 4 days was 2.2 mg/mL culture. The bioprocess using Acinetobacter sp. N-476-2 can be applied to the large-scale production of arachidonyl alcohol.







Similar content being viewed by others
References
Colin DF (2001) Prostaglandins and leukotrienes: advances in eicosanoid biology. Science 294:1871–1875
Carlson SE, Werkman SH, Peeples JM, Cooke RJ, Tolley EA (1993) Arachidonic acid status correlates with first year growth in preterm infants. Proc Natl Acad Sci USA 90:1073–1077
Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG (2000) A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol 42:174–181
Kotani S, Nakazawa H, Tokimasa T, Akimoto K, Kawashima H, Ono YT, Kiso Y, Okaichi H, Sakakibara M (2003) Synaptic plasticity preserved with arachidonic acid diet in aged rats. Neurosci Res 46:453–461
Stella N, Schweitzer P, Piomelli D (1997) A second endogenous cannabinoid that modulates long-term potentiation. Nature 388:773–778
Suhara Y, Takayama H, Nakane S, Miyashita T, Waku K, Sugiura T (2000) Synthesis and biological activities of 2-arachidonylglycerol, an endogenous cannabinoid receptor ligand, and its metabolically stable ether-linked analogues. Chem Pharm Bull 48:903–907
Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R (2001) 2-Arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci USA 98:3662–3665
Fezza F, Bisogno T, Minassi A, Appendino G, Mechoulam R, Marzo VD (2002) Noladin ether, a putative novel endocannabinoid: inactivation mechanisms and a sensitive method for its quantification in rat tissues. FABS Letters 513:294–298
Ryberg E, Vu HK, Larsson N, Groblewski T, Hjorth S, Elebring T, Sjogren S, Greasley PJ (2005) Identification and characterization of a novel splice variant of the human CB1 receptor. FEBS Lett 579:259–264
Juntunen J, Vepsalainen J, Niemi R, Laine K, Jarvinen T (2003) Synthesis, in vitro evaluation, and intraocular pressure effects of water-soluble prodrugs of endocannabinoid noladin ether. J Med Chem 46:5083–5086
Nagao T, Watanabe Y, Hiraoka K, Kishimoto N, Fujita T, Shimada Y (2009) Microbial conversion of vegetable oil to rare unsaturated fatty acids and fatty alcohols by an Aeromonas hydrophila isolate. J Am Oil Chem Soc 86:1189–1197
Nagao T, Shimada Y (2010) Microbial production of rare unsaturated fatty acids and alcohols. Lipid Technol 22:250–252
Yamada H, Shimizu S, Shinmem Y (1987) Production of arachidonic acid by Mortierella elongata 1S–5. Agric Biol Chem 51:780–790
Higashiyama K, Yaguchi T, Akimoto K, Fujikawa S, Shimizu S (1998) Enhancement of arachidonic acid production by Mortierella alpina 1S–4. J Am Oil Chem Soc 75:1501–1505
Sakuradani E, Ando A, Ogawa J, Shimizu S (2010) Arachidonic acid-producing Mortierella alpina: creation of mutants, isolation of the related enzyme genes, and molecular breeding. In: Cohen Z, Ratledge C (eds) Single cell oils: microbial and algal oils, 2nd edn. AOCS Press, Urbana, pp 29–49
Christie WW, Brechany EY, Johnson SB, Holman T (1986) A comparison of pyrrolidide and picolinyl ester derivatives for the identification of fatty acids in natural samples by gas chromatography-mass spectrometry. Lipids 21:657–661
Christie WW, Brechany EY, Holman T (1987) Mass spectra of the picolinyl esters of isomeric mono- and dienoic fatty acids. Lipids 22:224–228
Pfeffer PE, Luddy FE, Unruh J, Shoolery JN (1977) Analytical 13C NMR: a rapid, nondestructive method for determining the cis, trans composition of catalytically treated unsaturated lipid mixtures. J Am Oil Chem Soc 54:380–386
Sakai Y, Maeng JH, Tani Y, Kato N (1994) Use of long-chain n-alkanes (C13–C44) by an isolate, Acinetobacter sp. M-1. Biosci Biotech Biochem 58:2128–2130
Ishige T, Tani A, Takabe K, Kawasaki K, Sakai Y, Kato N (2002) Wax ester production from n-alkanes by Acinetobacter sp. strain M-1: ultrastructure of cellular inclusions and role of acyl coenzyme A reductase. Appl Environ Microbiol 68:1192–1195
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (No. 20580093) and a research grant from the Institute for Fermentation, Osaka, Japan.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nagao, T., Watanabe, Y., Tanaka, S. et al. Microbial Conversion of Arachidonic Acid to Arachidonyl Alcohol by a New Acinetobacter Species. J Am Oil Chem Soc 89, 1663–1671 (2012). https://doi.org/10.1007/s11746-012-2057-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-012-2057-3