Abstract
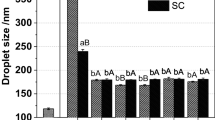
The effectiveness of various biopolymer emulsifiers at forming and stabilizing model beverage emulsions was examined: β-lactoglobulin (BLG); gum arabic (GA); conventional modified starch (MS-old); new modified starch (MS-new). Orange oil-in-water emulsions (5% oil) were prepared using high pressure homogenization. For BLG, MS-new, MS-old and GA, the minimum droplet diameters produced were 171, 254, 222 and 497 nm, while the minimum mass ratio of emulsifier-to-oil required to produce small droplets were 0.5:5, 1:5, 3:5 and 5:5, respectively. The influence of pH (3–8), ionic strength (0–500 mM NaCl, 0–50 mM CaCl2) and thermal treatment (30–90 °C) on the stability of the emulsions was examined. Extensive droplet aggregation occurred in BLG-stabilized emulsions around their isoelectric point (pH ≈ 5), at high salt concentrations (≥300 mM NaCl, ≥10 mM CaCl2, pH 7) and at high temperatures (>70 °C, 200 mM NaCl, pH 7) due to changes in electrostatic and hydrophobic interactions. There was little effect of pH, ionic strength and temperature on emulsions stabilized by GA or MS due to strong steric (rather than electrostatic) stabilization. The new type of modified starch used in this study was capable of forming stable emulsions with small droplet sizes at low concentrations.





Similar content being viewed by others
References
Given PS (2009) Encapsulation of flavors in emulsions for beverages. Curr Opin Colloid Interface Sci 14:43–47
McClements DJ (2005) Food emulsions: principles, practice, and techniques. CRC Press, Boca Raton
Tan C (1998) Beverage flavor emulsion—a form of emulsion liquid membrane microencapsulation. In: Contis ET (ed) Food flavors: formation, analysis and packaging influences. Elsevier, New York, p 29
Tan C (2004) Beverage emulsions. In: F. S, L. K and S. J Food emulsions. Marcel Decker, New York
Perez-Cacho PR, Rouseff RL (2008) Fresh squeezed orange juice odor: a review. Crit Rev Food Sci Nutr 48:681–695
Dong P, Qiu PJ, Zhu Y, Li SM, Ho CT, McClements DJ, Xiao H (2010) Simultaneous determination of four 5-hydroxy polymethoxyflavones by reversed-phase high performance liquid chromatography with electrochemical detection. J Chromatogr A 1217:642–647
Xiao H, Yang CS, Li SM, Jin HY, Ho CT, Patel T (2009) Monodemethylated polymethoxyflavones from sweet orange (Citrus sinensis) peel inhibit growth of human lung cancer cells by apoptosis. Mol Nutr Food Res 53:398–406
Manthey JA, Bendele P (2008) Anti-inflammatory activity of an orange peel polymethoxylated flavone. 3′,4′,3,5,6,7,8-heptamethoxyflavone, in the rat carrageenan/paw edema and mouse lipopolysaccharide-challenge assays. J Agric Food Chem 56:9399–9403
Walstra P (1993) Principles of emulsion formation. Chem Eng Sci 48:333
Walstra P (2003) Physical chemistry of foods. Marcel Decker, New York
Kralova I, Sjoblom J (2009) Surfactants used in food industry: a review. J Dispers Sci Technol 30:1363–1383
Brazel CS (1999) Microencapsulation: offering solutions for the food industry. Cereal Food World 44:388–393
Stauffer S (1999) Emulsifiers. Eagen Press, St Paul
Edris AE (1998) Preparation and stability of a protein stabilized orange oil-in-water emulsion. Nahrung Food 42:19–22
Trubiano PC (1995) The role of specialty food starches in flavor encapsulation. Flavor Technol 610:244–253
Garti N, Leser ME (2001) Emulsification properties of hydrocolloids. Polym Adv Technol 12:123–135
Garti N (1999) Hydrocolloids as emulsifying agents for oil-in-water emulsions. J Dispers Sci Technol 20:327–355
Klein M, Aserin A, Svitov I, Garti N (2010) Enhanced stabilization of cloudy emulsions with gum Arabic and whey protein isolate. Colloids Surf. B Biointerfaces 77:75–81
Chanamai R, McClements DJ (2002) Comparison of gum arabic, modified starch, and whey protein isolate as emulsifiers: Influence of pH, CaCl2 and temperature. J Food Sci 67:120–125
Harnsilawat T, Pongsawatmanit R, McClements DJ (2006) Stabilization of model beverage cloud emulsions using protein–polysaccharide electrostatic complexes formed at the oil–water interface. J Agric Food Chem 54:5540–5547
Tse KY, Reineccius GA (1995) Methods to predict the physical stability of flavor—cloud emulsion. In: Ho CT, Tan CT, Tong CH (eds) Flavor technology—physical chemistry, modification, and process, pp 172–182
Randall RC, Phillips GO, Williams PA (1988) The role of the proteinaceous component on the emulsifying properties of gum arabic. Food Hydrocolloids 2:131–140
Williams PA, Phillips GO, Randall RC (1990) Structure–function relationships of gum arabic. In: Gums and stabilisers for the food industry. Oxford University Press, Oxford, p 25
Phillips GO, Williams PA (2000) Gum arabic. In: Handbook of hydrocolloids. CRC Press, p 165
Stephen AM, Phillips GO, Williams PA (2005) Gums and mucilages. In: Food polysaccharides and their applications. Taylor & Francis, London, p 469
Jafari SM, Assadpoor E, He YH, Bhandari B (2008) Re-coalescence of emulsion droplets during high-energy emulsification. Food hydrocolloids 22:1191–1202
Tcholakova S, Denkov ND, Danner T (2004) Role of surfactant type and concentration for the mean drop size during emulsification in turbulent flow. Langmuir 20:7444–7458
Tcholakova S, Denkov ND, Sidzhakova D, Ivanov IB, Campbell B (2003) Interrelation between drop size and protein adsorption at various emulsification conditions. Langmuir 19:5640–5649
Kulmyrzaev A, Chanamai R, McClements DJ (2000) Influence of pH and CaCl2 on the stability of dilute whey protein stabilized emulsions. Food Res Int 33:15–20
Demetriades K, Coupland JN, McClements DJ (1997) Physical properties of whey protein stabilized emulsions as related to pH and NaCl. J Food Sci 62:342–347
Guzey D, McClements DJ (2007) Impact of electrostatic interactions on formation and stability of emulsions containing oil droplets coated by beta-lactoglobulin–pectin complexes. J. Agric. Food Chem. 55:475–485
Demetriades K, McClements DJ (1998) Influence of pH and heating on physicochemical properties of whey protein-stabilized emulsions containing a nonionic surfactant. J Agric Food Chem 46:3936–3942
Hu M, McClements DJ, Decker EA (2003) Impact of whey protein emulsifiers on the oxidative stability of salmon oil-in-water emulsions. J Agric Food Chem 51:1435–1439
McClements DJ, Decker EA (2000) Lipid oxidation in oil-in-water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems. J Food Sci 65:1270–1282
Acedo-Carrillo JI, Rosas-Durazo A, Herrera-Urbina R, Rinaudo M, Goycoolea FM, Valdez MA (2006) Zeta potential and drop growth of oil in water emulsions stabilized with mesquite gum. Carbohydr Polym 65:327–336
Israelachvili J (1992) Intermolecular and surface forces, 2nd edn. Academic Press, London
Kim HJ, Decker EA, McClements DJ (2002) Role of postadsorption conformation changes of beta-lactoglobulin on its ability to stabilize oil droplets against flocculation during heating at neutral pH. Langmuir 18:7577–7583
Kim HJ, Decker EA, McClements DJ (2005) Influence of protein concentration and order of addition on thermal stability of beta-lactoglobulin stabilized n-hexadecane oil-in-water emulsions at neutral pH. Langmuir 21:134–139
Kim HJ, Decker EA, McClements DJ (2004) Comparison of droplet Flocculation in hexadecane oil-in-water emulsions stabilized by beta-lactoglobulin at pH 3 and 7. Langmuir 20:5753–5758
Monahan FJ, McClements DJ, German JB (1996) Disulfide-mediated polymerization reactions and physical properties of heated WPI-stabilized emulsions. J Food Sci 61:504–509
Acknowledgments
The authors thank Jason Li (National Starch) for supplying the modified starch used in this study. This material is partly based upon work supported by United States Department of Agriculture, CREES, NRI Grants, and Massachusetts Department of Agricultural Resources. We also acknowledge funding from the University of Massachusetts (Hatch).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Qian, C., Decker, E.A., Xiao, H. et al. Comparison of Biopolymer Emulsifier Performance in Formation and Stabilization of Orange Oil-in-Water Emulsions. J Am Oil Chem Soc 88, 47–55 (2011). https://doi.org/10.1007/s11746-010-1658-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-010-1658-y