Abstract
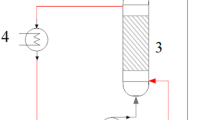
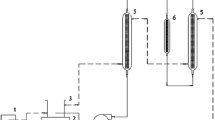
Adsorption isotherms for a packed column consisting of fructose and silica gel in contact with a liquid phase consisting of oleic acid and fructose mono- and di-oleate at 65 °C were determined to assist the column’s utilization in a bioreactor system for the solvent-free lipase-catalyzed synthesis of saccharide ester biosurfactants. The liquid-phase fructose content was very low, ranging from 0.005 to 0.1% (w/w), with the higher values obtained for a higher fructose concentration in the column and a higher ester content in the liquid phase. The desorption behavior of fructose followed the Freundlich isotherm. An increase in the liquid phase space velocity increased the initial rate of fructose desorption linearly. Dynamic light scattering measurements demonstrate solutions obtained by mechanical stirring of crystalline fructose and oleic acid/fructose oleate contained 50–250 μm sized aggregates, even after centrifugation at 12,000 rpm, but that the aggregates were absent from the desorption column effluent stream. Therefore, employment of a desorption column will lead to a low saccharide concentration in the liquid phase, but will reduce suspension-induced fouling of lipase in a bioreactor downstream of the column, which could improve the retention of lipase activity.





Similar content being viewed by others
References
Nakamura S (1997) Using sucrose esters as food additives. Inform 8:866–874
Szuts A, Pallagi E, Regdon G Jr, Aigner Z, Szabo-Revesz P (2007) Study of thermal behaviour of sugar esters. Int J Pharm 336:199–207
Feuge RO, Zeringue HJ Jr, Weiss TJ, Brown M (1970) Preparation of sucrose esters by interesterification. J Am Oil Chem Soc 47:56–60
Hoydonckx HE, De Vos DE, Chavan SA, Jacobs PA (2004) Esterification and transesterification of renewable chemicals. Top Catal 27:83–96
Vulfson E (2003) Enzymatic synthesis of surfactants. In: Holmberg K (ed) Novel surfactants. Marcel-Dekker, New York, pp 257–278
Ballesteros A, Plou FJ, Alcalde M, Ferrer M, Garcia-Arellano H, Reyes-Duarte D, Ghazi I (2007) Enzymatic synthesis of sugar esters and oligosaccharides from renewable resources. In: Patel RN (ed) Biocatalysis in the pharmaceutical and biotechnology industries. CRC Press, Boca Raton, pp 463–488
Sarney DB, Vulfson EN (2001) Enzymatic synthesis of sugar fatty acid esters in solvent-free media. In: Vulfson EN, Halling PJ, Holland HL (eds) Enzymes in nonaqueous solvents: methods and protocols. Methods in biotechnology, vol 15. Humana Press, Totawa, pp 531–543
Villeneuve P (2007) Lipases in lipophilization reactions. Biotechnol Adv 25:515–536
Adachi S, Kobayashi T (2005) Synthesis of esters by immobilized-lipase-catalyzed condensation reaction of sugars and fatty acids in water-miscible organic solvent. J Biosci Bioeng 99:87–94
Dang HT, Obiri OO, Hayes DG (2005) Feed batch addition of saccharide during saccharide-fatty acid esterification catalyzed by immobilized lipase: time course, water activity, and kinetic model. J Am Oil Chem Soc 82:487–493
Obiri OO (2006) Synthesis of lipase-catalyzed saccharide fatty acid esters using a packed bed bioreactor system with continuous re-circulation of reaction medium: a continuation of batch-mode-related research. Masters Thesis, Department of Biosystems Engineering & Soil Science, University of Tennessee, Knoxville, TN
Nowak J, Gedicke K, Antos D, Piatkowski W, Seidel-Morgenstern A (2007) Synergistic effects in competitive adsorption of carbohydrates on an ion-exchange resin. J Chromatogr A 1164:224–234
Gramblicka M, Polakovic M (2007) Adsorption equilibria of glucose, fructose, sucrose, and fructooligosaccharides on cation exchange resins. J Chem Eng Data 52:345–350
Kawajiri Y, Biegler LT (2006) Optimization strategies for simulated moving bed and power feed processes. AIChE J 52:1343–1350
Bubnik Z, PourGruberova ZA, Sterhova H, Hlinkova A, Kadlec P (2004) Application of continuous chromatographic separation in sugar processing. J Food Eng 61:509–513
Yu C-M, Mun S, Wang N-HL (2006) Theoretical analysis of the effects of reversible dimerization in size exclusion chromatography. J Chromatogr A 1132:99–108
Freundlich H (1907) Ueber die adsorption in Iosungen. Z Physik Chem 57:385–470
Zhang X, Hayes DG (1999) Increased rate of lipase-catalyzed saccharide–fatty acid esterification by control of reaction medium. J Am Oil Chem Soc 76:1495–1500
Acknowledgments
Financial support was kindly supplied by the US Department of Agriculture, Grant 2006-35504-17262. We also thank Dr. Svetlana Zivanovic and Ms. Jennifer Marie Ham for their assistance in the analysis of particle size distribution.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Pyo, SH., Hayes, D.G. Desorption of Fructose from a Packed Column to an Oleic Acid/Fructose Oleate Mixture for Employment in a Bioreactor System. J Am Oil Chem Soc 85, 1033–1040 (2008). https://doi.org/10.1007/s11746-008-1302-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-008-1302-2