Abstract
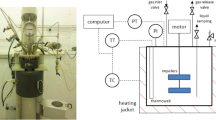
Biodiesel is made by the transesterification of vegetable oils to form alkyl FA esters. High levels of conversion (>99%) are required to lower the total concentration of free and chemically bound glycerol to that allowed by the ASTM standard (0.240 wt%) for biodiesel. A polar dye was used to visualize the phase behaviors in methanolysis, ethanolysis, and butanolysis. The dye was more strongly colored in more polar phases. Methanolysis and ethanolysis reactions commenced as two phases (alcohol and oil), then formed emulsions, and ended as two phases as glycerol-rich phases separated. Ethanolysis was more easily initiated by mixing than was methanolysis. Ethanolysis also exhibited a much longer emulsion period and slower glycerol separation. The glycerol-rich phase was smaller in volume in ethanolysis than in methanolysis. Butanolysis remained one phase throughout, and no polar phase existed at any time. The results are consistent with the known phase compositions in these reactions. The concentrations of MG, DG, and TG in the products with time in stirred reactions were consistent with the observed phase behavior in the dye experiments.
Similar content being viewed by others
References
Freedman, B., F. Pryde, and T.J. Mounts, Variables Affecting the Yields of Fatty Esters from Transesterified Vegetable Oils, J. Am. Oil Chem. Soc., 61:1638–1643 (1984).
Freedman, B., R.O. Butterfield, and E.H. Pryde, Transesterification Kinetics of Soybean Oil, ——Ibid. 63:1375–1380 (1986).
Darnoko, D., and M. Cheryan, Kinetics of Palm Oil Transesterification in a Batch Reactor, ——Ibid., 77:1263–1267 (2000).
Boocock, D.G.B., S.K. Konar, V. Mao, C. lee, and S. Buligan, Fast Formation of High Purity Methyl Esters from Vegetable Oils, ——Ibid. 75:1167–1172 (1998).
Boocock, D.G.B., S.K. Konar, G.V. Balkansky, L.R. Chi, W.Y. Zhou, and J. Lutzen, Ambient Pressure Technologies for the Production of Biodiesel Methyl Esters, Proceedings of the First World Conference and Exhibition on Biomass for Energy and the Environment, Vol. 1, James & James, London, 2001, pp. 590–592.
Zhou, W., S.K. Konar, and D.B.G. Boocock, Ethyl Ester from Single-Phase Base-Catalyzed Ethanolysis of Vegetable Oils, J. Am. Oil Chem. Soc. 80:367–371 (2003).
ASTM D 6584, Standard Test Method for Determination of Free and Total Glycerine in B-100 Biodiesel Methyl Esters by Gas Chromatography, ASTM International, West Conschohocken, PA, 2000
Zhou, W., and D.G.B. Boocock, Phase Distribution of Alcohol, Glycerol, and Catalyst in the Transesterification of Triglycerides, J. Am. Oil Chem. Soc. 83:xxx-xxx (2006).
Korus, A.R., D.S. Hoffman, N. Bam, C.L. Peterson, and D.C. Drown, Transesterification Process to Manufacture Ethyl Ester of Rape Oil, First Biomass Conference of the Americas: Energy, Environment, Agriculture, and Industry, Vol. 2, August 30–September 2, 1993, Burlington, Vermont, 1993, National Renewable Energy Laboratory, Golden, Colorado, Vol. 2, pp. 815–822.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Zhou, W., Boocock, D.G.B. Phase behavior of the base-catalyzed transesterification of soybean oil. J Amer Oil Chem Soc 83, 1041–1045 (2006). https://doi.org/10.1007/s11746-006-5160-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11746-006-5160-5