Abstract
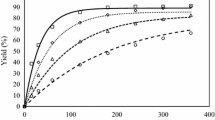
The present work focused on the glycerolysis of fatty acid methyl esters. The aim was to develop and test a kinetic model that could be used to reliably simulate different process alternatives for this reaction. A prerequisite was the identification and characterization of the factors that affect the reaction kinetics. Experiments were carried out in a batch reactor with and without forced removal of methanol, which is one of the reaction products. Concentrations of all components in the two-phase system were measured. It was found that the methanol concentration has a strong effect on the reaction rate and equilibrium conversion. Near-complete conversions were obtained by stripping methanol with an inert gas. The glycerol concentration in the ester phase was found to increase as the reaction proceeds, which also accelerates the reaction. Effects of mass transfer on the reaction rate were not found to control the reaction rate under well-agitated conditions. A semi-empirical model was used to simulate the reaction. The results from the semi-empirical model show good agreement with experimental results.





Similar content being viewed by others
References
Henry C (1995) Monoglycerides: the universal emulsifier. Cereal Foods World 40(10):734–738
Meffert A (1984) Technical uses of fatty acid esters. J Am Oil Chem Soc 61(2):255–258
Sonntag N (1992) Glycerolysis of fats and methyl esters—status, review and critique. J Am Oil Chem Soc 50(10):795–802
Jeromin L, Wozny G, Li P (2000) US Patent 6,127,561
Bossaert WD, De Vos DE, Van Rhijn WM, Bullen J, Grobet PJ, Jacobs PA (1999) Mesoporous sulfonic acids as selective heterogeneous catalysts for the synthesis of monoglycerides. J Catal 182:156–164
Noureddini H, Medikonduru V (1997) Glycerolysis of fats and methyl esters. J Am Oil Chem Soc 74(4):419–425
Noureddini H, Harkey D, Medikonduru V (1998) A continuous process for the conversion of vegetable oils into methyl esters of fatty acids. J Am Oil Chem Soc 75(12):1775–1783
Noureddini H, Harkey DW, Gutsman MR (2004) A continuous process for the glycerolysis of soybean oil. J Am Oil Chem Soc 81(2):203–207
Freedman B, Butterfield RO, Pryde EH (1986) Transesterification kinetics of soybean oil. J Am Oil Chem Soc 63(10):1375–1380
Noureddini H, Zhu D (1997) Kinetics of transesterification of soybean oil. J Am Oil Chem Soc 74(11):1457–1463
Lotero E, Liu Y, Lopez DE, Suwannakarn K, Bruce DA, Goodwin JG Jr (2005) Synthesis of biodiesel via acid catalysts. Ind Eng Chem Res 44(14):5353–5363
Rodger WA, Trice VG, Rushton JH Jr (1956) Effect of fluid motion on interfacial areas of dispersions. Chem Eng Prog 52(12):515–520
Kimmel T (2004) Kinetic investigation of the base-catalyzed glycerolysis of fatty acid methyl esters. PhD Thesis, Institut für Chemie, TU Berlin, Germany
Negi DS, Sobotka F, Kimmel T, Wozny G, Schomäcker R (2006) Liquid–liquid phase equilibrium in glycerol–methanol–methyl oleate and glycerol–monoolein–methyl oleate ternary systems. Ind Eng Chem Res 45(10):3693–3696
Fredenslund A, Jones RL, Prausnitz JM (1975) Group-contribution estimation of activity coefficients in nonideal liquid mixtures. AIChE J 21(6):1086–1099
Weidlich U, Gmehling J (1987) A modified UNIFAC model. 1. Prediction of VLE, h e and γ ∞. Ind Eng Chem Res 26:1372–1381
Negi DS (2006) Base-catalyzed glycerolysis of fatty acid methyl esters: investigations towards the development of a continuous process. PhD Thesis, Institut für Chemie, TU Berlin, Germany
Cussler EL (2003) Diffusion: mass transfer in fluid systems, 2nd edn. Cambridge University Press, New York
Kimmel T, Joshi M, Schomäcker R (2002) Determination of drop sizes through microphotography in reactive liquid–liquid system with high dispersed phase fraction (in German). Chem Ing Tech 74(9):1281–1285
Negi DS, Rochlitz A, Wozny G, Schomäcker R (2005) Drop-size analysis in a two-phase reactive liquid–liquid system on a bubble-cap tray. Ind Eng Chem Res 44(9):3343–3347
Perry RH, Green DW (1997) Perry’s chemical engineers’ handbook, 7th edn. McGraw-Hill, New York, Ch 5
Acknowledgments
The authors wish to acknowledge the Berliner Graduiertenkolleg 827 “Transportvorgänge an bewegten Phasengrenzflächen” and the Deutsche Forschungsgemeinschaft (DFG) for financial support. One of the authors (Negi) wishes to thank Y. Kasaka for help with the experimental work.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Negi, D.S., Sobotka, F., Kimmel, T. et al. Glycerolysis of Fatty Acid Methyl Esters: 1. Investigations in a Batch Reactor. J Amer Oil Chem Soc 84, 83–90 (2007). https://doi.org/10.1007/s11746-006-1009-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-006-1009-1