Abstract
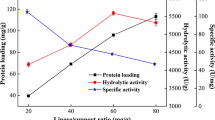
TAG (MLM) with medium-chain FA (MCFA) at the 1,3-positions and long-chain FA (LCFA) at the 2-position, and TAG (LMM) with LCFA at the 1(3)-position and MCFA at 2,3(1)-positions are a pair of TAG regioisomers. Large-scale preparation of the two TAG regioisomers was attempted. A commercially available FFA mixture (FFA-CLA) containing 9-cis, 11-trans (9c, 11t)- and 10t,12c-CLA was selected as LCFA, and caprylic acid (C8FA) was selected as MCFA. The MLM isomer was synthesized by acidolysis of acyglycerols (AG) containing two CLA isomers with C8FA: A mixture of AG-CLA/C8 FA (1∶10, mol/mol) and 4 wt% immobilized Rhizomucor miehei lipase was agitated at 30°C for 72 h. The ratio of MLM to total AG was 51.1 wt%. Meanwhile, LMM isomer was synthesized by acidolysis of tricaprylin with FFA-CLA: A mixture of tricaprylin/FFA-CLA (1∶2, mol/mol) and 4 wt% immobilized R. miehei lipase was agitated at 30°C for 24 h. The ratio of LMM to total AG was 51.8 wt%. MLM and LMM were purified from 1,968 and 813 g reaction mixtures by stepwise short-path distillation, respectively. Consequently, MLM was purified to 92.3% with 49.1% recovery, and LMM was purified to 93.2% with 52.3% recovery. Regiospecific analyses of MLM and LMM indicated that the 2-positions of MLM and LMM were 95.1 mol% LCFA and 98.3 mol% C8 FA, respectively. The results showed that a process comprising lipase reaction and short-path distillation is effective for large-scale preparation of high-purity regiospecific TAG isomers.
Similar content being viewed by others
References
Akoh, C.C., K.T. Lee, and L. Fomuso, Synthesis of Positional Isomers of Structured Lipids with Lipases as Biocatalysts, in Structural Modified Food Fats: Synthesis, Biochemistry, and Use, edited by A.B. Christophe, AOCS Press, Champaign, 1998, pp. 46–72.
Xu, X., A.R.H. Skands, C.-E. Høy, H. Mu, S. Balchen, and J. Adler-Nissen, Production of Specific-Structured Lipids by Enzymatic Interesterification: Elucidation of Acyl Migration by Response Surface Design, J. Am. Oil Chem. Soc. 75:1179–1186 (1998).
Han, J.J., and T. Yamane, Enhancement of Both Reaction Yield and Rate of Synthesis of Structured Triacylglycerol Containing Eicosapentaenoic Acid Under Vacuum with Water Activity Control, Lipids 34:989–995 (1999).
Xu, X., Enzymatic Production of Structured Lipids: Process Reactions and Acyl Migration, inform 11:1121–1131 (2000).
Shimada, Y., A. Sugihara, and Y. Tominaga, Production of Functional Lipids Containing Polyunsaturated Fatty Acids with Lipase, in Enzymes in Lipid Modification, edited by U.T. Bornscheuer, Wiley-VCH, Weinheim, Germany, 2000, pp. 128–147.
Kawashima, A., Y. Shimada, M. Yamamoto, A. Sugihara, T. Nagao S. Komemoshi, and Y. Tominaga, Enzymatic Synthesis of High-Purity Structured Lipids with Caprylic Acid at 1,3-Positions and Polyunsaturated Fatty Acid at 2-Position, J. Am. Oil Chem. Soc. 78:611–616 (2001).
Mu, H., J.-P. Kurvinen, H. Kallio, X. Xu, and C.-E. Høy, Quantitation of Acyl Migration During Lipase-Catalyzed Acidolysis and of the Regioisomers of Structured Triacylglycerols Formed, 78:959–964 (2001).
Xu, X., A. Skands, and J. Adler-Nissen, Purification of Specific Structured Lipids by Distillation: Effects on Acyl Migration, 78:715–718 (2001).
Negishi, S., Y. Arai, S. Arimoto, K. Tsuchiya, and I. Takahashi, Synthesis of 1,3-Dicapryloyl-2-docasahexaenoylglycerol by a Combination of Nonselective and sn-1,3-Selective Lipase Reactions, 80:971–974 (2003).
Shimada, Y., A. Sugihara, K. Maruyama, T. Nagao, S. Nakayama, H. Nakano, and Y. Tominaga, Production of Structured Lipid Containing Docosahexaenoic and Caprylic Acids Using Immobilized Rhizopus delemar Lipase, J. Ferment. Bioeng. 81:299–303 (1996).
Irimescu, R., M. Yasui, Y. Iwasaki, N. Shimidzu, and T. Yamane, Enzymatic Synthesis of 1,3-Dicapryloyl-2-eicosapentaenoylglycerol, J. Am. Oil Chem. Soc. 77:501–506 (2000).
Mu, H., and C.-E. Høy, Effect of Different Medium-Chain Fatty Acids on Intestinal Absorption of Structured Triacylglycerols, Lipids 35:83–89 (2000).
Hamosh, M., and P. Hamosh, Specificity of Lipases: Developmental Physiology with Aspects—The Role of Lipase Selectivity in the Digestion of Milk Fat, in Enzyme Engineering of/with Lipases, edited by F.X. Malcata, Kluwer, Dordrecht, The Netherlands, 1996, pp. 31–49.
Iverson, S.J., C.L. Kirk, M. Harmosh, and J. Newsome, Milk Lipid Digestion in the Neonatal Dog: The Combined Actions of Gastric and Bile Salt Stimulated Lipases, Biochim. Biophys. Acta 1083:109–119 (1991).
Christensen, M.S., C.-E. Høy, C.C. Becker, and T.G. Redgrave, Intestinal Absorption and Lymphatic Transport of Eicosapentaenoic (EPA), Docosahexaenoic (DHA), and Decanoic Acids: Dependence on Intramolecular Triacylglycerol Structure, Am. J. Clin. Nutr. 61:56–61 (1995).
Pariza, M.W., CLA, A New Cancer Inhibitor in Dairy Products, Bull. Int. Dairy Fed. 257:29–30 (1991).
Ha, Y.L., N.K. Grimm, and M.W. Pariza, Anticarcinogens from Fried Ground Beef: Heat-Altered Derivatives of Linoleic Acid, Carcinogenesis 8:1881–1887 (1987).
Park, Y., K.J. Albright, W. Liu, J.M. Storkson, M.E. Cook, and M.W. Pariza, Effect of Conjugated Linoleic Acid on Body composition in Mice, Lipids 32:853–858 (1997).
Ostrowska, E., M. Muralitharan, R.F. Cross, D.E. Bauman, and F.R. Dunshea, Dietary Conjugated Linoleic Acids Increase Lean Tissue and Decrease Fat Deposition in Growing Pigs, J. Nutr. 129:2037–2042 (1999).
Rahman, S.M., Y.-M. Wang, H. Yotsumoto, J.-Y. Cha, S.-Y. Han, S. Inoue, and T. Yanagita, Effect of Conjugated Linoleic Acid on Serum Leptin Concentration, Body-Fat Accumulation, and β-Oxidation of Fatty Acid in OLETF Rats, Nutrition 17:385–390 (2001).
Lee, K.N., D. Kritchevsky, and M.W. Pariza, Conjugated Linoleic Acid and Atherosclerosis in Rabbits, Atherosclerosis 108:19–25 (1994).
Nicolosi, R.J., E.J. Rogers, D. Kritchevsky, J.A. Scimeca, and P.J. Huth, Dietary Conjugated Linoleic Acid Residues Plasma Lipoproteins and Early Aortic Atherosclerosis in Hypercholesterolemic Hamsters, Artery 22:266–277 (1997).
Sugano, M., A. Tsujita, M. Yamasaki, M. Noguchi, and K. Yamada, Conjugated Linoleic Acid Modulates Tissue Levels of Chemical Mediators and Immunoglobulins in Rats, Lipids 33:521–527 (1998).
Yamauchi-Sato, Y., T. Nagao, T. Yamamoto, T. Terai, A. Sugihara, and Y. Shimada, Fractionation of Conjugated Linoleic Acid Isomers by Selective Hydrolysis with Candida rugosa Lipase, J. Oleo Sci. 52:367–374 (2003).
Nagao, T., Y. Shimada, Y. Yamauchi-Sato, T. Yamamoto, M. Kasai, K. Tsutsumi, A. Sugihara, and Y. Tominaga, Fractionation and Enrichment of CLA Isomers by Selective Esterification with Candida rugosa Lipase, J. Am. Oil Chem. Soc. 79:303–308 (2002).
Shimada, Y., J. Ogawa, Y. Watanabe, T. Nagao, A. Kawashima, T. Kobayashi, and S. Shimizu, Regiospecific Analysis by Ethanolysis of Oil with Immobilized Candida antarctica Lipase, Lipids 38:1281–1286 (2003).
Kawashima, A., Y. Shimada, T. Nagao, A. Ohara, T. Matsuhisa, A. Sugihara, and Y. Tominaga, Production of Structured TAG Rich in 1,3-Dicapryloyl-2-γ-linolenoyl Glycerol from Borage Oil, J. Am. Oil Chem. Soc. 79:871–877 (2002).
Nagao, T., A. Kawashima, M. Sumida, Y. Watanabe, K. Akimoto, H. Fukami, A. Sugihara, and Y. Shimada, Production of Structured TAG Rich in 1,3-Dicapryloyl-2-arachidonoyl Glycerol from Mortierella Single-Cell Oil, 79:871–877 (2002).
Becker, C.C., A. Rosenquist, and G. Hølmer, Regiospecific Analysis of Triacylglycerols Using Allyl Magnesium Bromide, Lipids 28:147–149 (1993).
Luddy, F.E., R.A. Barford, S.F. Herb, P. Magidman, and R.W. Riemenschneider, Pancreatic Lipase Hydrolysis of Triglycerides by a Semimicro Technique, J. Am. Oil Chem. Soc. 41:693–696 (1964).
Kallio, H., and G. Currie, Analysis of Natural Fats and Oils by Ammonia Negative Ion Tandem Mass Spectrometry—Triacylglycerols and Positional Distribution of Their Acyl Groups, in CRC Handbook of Chromatography, Analysis of Lipids, edited by K.D. Mukherjee and N. Weber, CRC Press, Boca Raton, Florida, 1993, pp. 435–458.
Irimescu, R., Y. Iwasaki, and C.T. Hou, Study of TAG Ethanolysis to 2-MAG by Immobilized Candida antarctica Lipase and Synthesis of Symmetrically Structured TAG, J. Am. Oil Chem. Soc. 79:879–883 (2002).
Irimescu, R., K. Furihata, K. Hata, Y. Iwasaki, and T. Yamane, Utilization of Reaction Medium-Dependent Regiospecificity of Candida antarctica Lipase (Novozym 435) for the Synthesis of 1,3-Dicapryloyl-2-docosahexaenoyl (or eicosapentaenoyl) Glycerol, 78:285–289 (2001).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kawashima, A., Nagao, T., Watanabe, Y. et al. Preparation of regioisomers of structured TAG consisting of one mole of CLA and two moles of caprylic acid. J Amer Oil Chem Soc 81, 1013–1020 (2004). https://doi.org/10.1007/s11746-004-1015-3
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11746-004-1015-3