Abstract
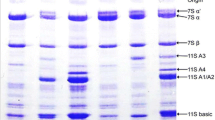
Glycinin (11S) and β-conglycinin (7S) are important seed storage proteins in soybean [Glycine max (L.) Merr.]. A major limitation of soybean seed storage proteins is their low levels of the sulfur-containing amino acids, methionine and cysteine, which are important nutritional components of protein mea. Glycinin contains significantly more S-containing amino acids than does β-conglycinin. Thus, detection of quantitative trait loci (QTL) that govern 11S may provide marker-assisted selection (MAS) opportunities to improve soybean total S-containing amino acids. The objective of this study was to detect and map QTL governing 7S and 11S fractions of soybean seed storage proteins. To achieve this objective, 101 F6-derived recombinant inbred lines (RIL) developed from a cross of N87-984-16 ×TN93-99 were used. Storage proteins were extracted from all RIL and separated in 10–20% linear gradient polyacrylamide gels. Dried gels were scanned for individual subunits of storage protein with a densitometer equipped with a He−Ne laser light source. Data were converted to concentration for each subunit component and analyzed using SAS software. A significant (P<0.05) difference among genotypes was found for glycinin and β-conglycinin. A total of 94 polymorphic simple sequence repeat molecular genetic markers were used in screening all RIL. Three QTL for glycinin (Satt461, Satt292, and Satt156) were distributed on linkage group (LG) D2, I, and L, respectively, whereas two QTL for conglycinin (Satt461 and Satt249) were distributed on LG D2 and J. Phenotypic variation explained by individual QTL ranged from 9.5 to 22%. These QTL may provide useful MAS opportunities for improvement of nutritional quality in soybean.
Similar content being viewed by others
References
Clarke, E.J., and J. Wiseman, Developments in Plant Breeding for Improved Nutritional Quality of Soybeans I. Protein and Amino Acids Content, J. Agric. Sci. 134:111–124 (2000).
Tsukada, Y., K. Kitamura, K. Harada, and N. Kaizuma, Genetic Analysis of Two Major Sub-units (β-conglycinin and glycinin) in Soybean Seeds, Jpn. J. Soybean 36:390–400 (1986).
Yagasaki, K., T. Takagi, M. Sakai, and K. Kitamura, Biochemical Characterization of Soybean Protein Consisting of Different Subunits of Glycinin, J. Agric. Food Chem. 45:656–660 (1997).
Kitamura, K., Genetic Improvement of Nutrition and Food Processing Quality in Soybean, Jpn. Agric. Res. Quart 29:1–8 (1995).
Ogawa, T., E. Tayama, K. Kitamura, and N. Kaizama, Genetic Improvement of Seed Storage Proteins Using Three Variant Alleles of 7S Globulin Sub-Unit in Soybean (Glycine max L.), Jpn. J. Breeding 39:137–147 (1989).
Kitagawa, S., M. Ishimoto, F. Kikuchi, and K. Kitamura, A Characteristic Lacking or Decreasing Remarkably 7S Globulin Sub-unit Induced with X-Ray Irradiation in Soybean Seeds, 41:460–461 (1991).
Takahashi, K., Y. Mizumo, S. Yumoto, K. Kitamura, and S. Nakamura, Inheritance of the α-Sub-unit Deficiency of β-Conglycinin in Soybean (Glycine max L.) Line Induced by γ-Ray Irradiation, Breeding Sci. 46:251–255 (1996).
Yaklich, R.W., β-Conglycinin and Glycinin in High-Protein Soybean Seeds, J. Agric. Food Chem. 49:729–735 (2001).
Nielsen, N.C., C.D. Dickinson, T.J. Cho, V.H. Thanh, B.J. Scallon, R.L. Fischer, T.L. Sims, G.N. Drews, and R.B. Goldberg, Characterization of the Glycinin Gene Family in Soybean, Plant Cell 1:313–328 (1989).
Harada, J.J., S.J. Barker, and R.B. Goldberg, Soybean β-Conglycinin Genes Are Clustered in Several DNA Regions and Are Regulated by Transcriptional and Posttranscriptional Processes, 1:415–425 (1989).
Hayashi, M., M. Nishioka, K. Kitamura, and K. Harada, Identification of AFLP Markers Tightly Linked to the Gene for Deficiency of the 7S Globulin in Soybean Seed and Characterization of Abnormal Phenotypes Involved in the Mutation, Breeding Sci. 50:123–129 (2000).
Kitamura, K., and N. Kaizuma, Mutant Strains with Low Level of Subunits of 7S Globulin in Soybean (Glycine max Merr.) Seed, Jpn. J. Breeding 31:353–359 (1981).
Mandal, S., and R. Mandal, Seed Storage Proteins and Approaches for Improvement of Their Nutritional Quality by Genetic Engineering, Curr. Sci. 79:576–589 (2000).
Kwanyuen, P., V.R. Pantalone, J.W. Burton, and R.F. Wilson, A New Approach to Genetic Alteration of Soybean Protein Composition and Quality, J. Am. Oil Chem. Soc. 74:983–987 (1997).
Liu, K., Soybeans: Chemistry, Technology and Utilization, Chapman and Hall, London, 1997, pp. 478–523.
Burton, J.W., T.E. Carter, and R.F. Wilson, Registration of Prolina Soybean, Crop Sci. 39:294–295 (1999).
Luck, P., T. Lanier, C. Daubert, R.F. Wilson, and P. Kwanyuen, Functionality and Visoelastic Behavior of Prolina Soybean Isolate, in Oilseed Processing and Utilization, edited by R.F. Wilson, AOCS Press, Champaign, 2001, pp. 197–202.
Pantalone, V.R., F.L. Allen, and D. Landau-Ellis, Registration of ‘Tn93-99’ Soybean Germplasm, Crop Sci. 43:1137 (2003).
Bradford, M.M., A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding, Anal. Biochem. 2:248–254 (1976).
Chua, N.H., Electrophoretic Analysis of Chloroplast Proteins, Methods Enzymol. 69:434–446 (1980).
Kwanyuen, P., and R.F. Wilson, Optimization of Coomassie Staining for Quantitative Densitometry of Soybean Storage Proteins in Gradient Gel Electrophoresis, J. Am. Oil Chem. Soc. 77:1251–1254 (2000).
Cregan, P.B., T. Jarvik, A.L. Bush, R.C. Shoemaker, K.G. Lark, A.L. Kahler, N. Kaya, T.T. VanToai, D.G. Lohnes, and J. Chung, An Integrated Genetic Linkage Map of the Soybean Genome, Crop Sci. 39:1464–1490 (1999).
SAS Guide for Personal Computers, SAS Institute, Cary, NC, 2000.
Krishnan, H.B., Biochemistry and Molecular Biology of Soybean Seed Storage Proteins, J. New Seeds 2:1–25 (2000).
Wilson, R.F., Current Research on Oilseeds, in Institute of Food Technologists 1996 Symposium: Identity-Preserved Oils, symposium held at the annual meeting of the Institute of Food Technologists, New Orleans, 1996.
So, E., Y. Chae, Y. Kim, and Y. MH, Variation of 7S and 11S Seed Protein Concentrations in Different Food Types of Soybean Seed, Korean J. Crop Sci. 44:350–354 (1999).
Fehr, W.R., J.A. Hoeck, S.L. Johnson, P.A. Murphy, J.D. Nott, G.I. Padilla, and G.A. Welke, Genotype and Environment Influence on Protein Components of Soybean, Crop Sci. 43:511–514 (2003).
Brummer, E.C., G.L. Graef, J. Orf, J.R. Wilcox, and R.C. Shoemaker, Mapping QTL for Seed Protein and Oil Content in Eight Soybean Populations, 37:370–378 (1997).
Stuber, C., S. Lincoln, D. Wolff, T. Helentjaris, and E. Lander, Identification of Genetic Factors Contributing to Heterosis in a Hybrid from Two Elite Maize Inbred Lines Using Molecular Markers, Genetics, 132:823–839 (1992).
Qiu, B.X., P.R. Arelli, and D.A. Sleper, RFLP Markers Associated with Soybean Cyst Nematode Resistance and Seed Composition in a ‘Peking’בEssex’ Population, Theor. Appl. Genet. 98:356–364 (1999).
Cho, T.J., C.S. Davies, and N.C. Nielsen, Inheritance and Organization of Glycinin Genes in Soybean, Plant Cell 1:329–337 (1989).
Beilinson, V., Z. Chen, R.C. Shoemaker, R.L. Fischer, R.B. Goldberg, and N.C. Nielsen, Genomic Organization of Glycinin Genes in Soybean, Theor. Appl. Genet. 104:1132–1140 (2002).
Nielsen, N., Soybean Seed Composition, in Soybean: Genetics, Molecular Biology and Biotechnology, edited by D. Verma, and R. Shoemaker, CAB International, Wallingford, 1996, pp. 127–163.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Panthee, D.R., Kwanyuen, P., Sams, C.E. et al. Quantitative trait loci for β-conglycinin (7S) and glycinin (11S) fractions of soybean storage protein. J Amer Oil Chem Soc 81, 1005–1012 (2004). https://doi.org/10.1007/s11746-004-1014-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11746-004-1014-4