Abstract
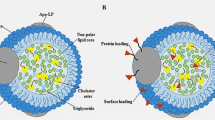
Lipid core nanoparticles (LDE) resembling LDL behave similarly to native LDL when injected in animals or subjects. In contact with plasma, LDE acquires apolipoproteins (apo) E, A-I and C and bind to LDL receptors. LDE can be used to explore LDL metabolism or as a vehicle of drugs directed against tumoral or atherosclerotic sites. The aim was to investigate in knockout (KO) and transgenic mice the plasma clearance and tissue uptake of LDE labeled with 3H-cholesteryl ether. LDE clearance was lower in LDLR KO and apoE KO mice than in wild type (WT) mice (p < 0.05). However, infusion of human apoE3 into the apoE KO mice increased LDE clearance. LDE clearance was higher in apoA-I KO than in WT. In apoA-I transgenic mice, LDE clearance was lower than in apoA-I KO and than in apoA-I KO infusion with human HDL. Infusion of human HDL into the apoA-I KO mice resulted in higher LDE clearance than in the apoA-I transgenic mice (p < 0.05). In apoA-I KO and apoA-I KO infused human HDL, the liver uptake was greater than in WT animals and apoA-I transgenic animals (p < 0.05). LDE clearance was lower in apoE/A-I KO than in WT. Infusion of human HDL increased LDE clearance in those double KO mice. No difference among the groups in LDE uptake by the tissues occurred. In conclusion, results support LDLR and apoE as the key players for LDE clearance, apoA-I also influences those processes.



Similar content being viewed by others
Abbreviations
- 3H–CE:
-
3H-cholesteryl ether
- apoA-I:
-
Apolipoprotein A-I
- apoB100:
-
Apolipoprotein B100
- apoE/A-I:
-
Apolipoprotein E/apolipoprotein A-I
- apoE3:
-
Apolipoprotein E3
- apoE:
-
Apolipoprotein E
- KO:
-
Knockout
- LDL:
-
Low density lipoprotein
- LDLR:
-
Low density lipoprotein receptor
- SR-BI:
-
Scavenger receptor class B type I
- WT:
-
Wild type
References
Maranhão RC, Cesar TB, Pedroso-Mariani SR, Hirata MH, Mesquita CH (1993) Metabolic behavior in rats of a nonprotein microemulsion resembling low-density lipoprotein. Lipids 28:691–696
Maranhão RC, Roland IA, Toffoletto O, Ramires JA, Gonçalves RP, Mesquita CH, Pileggi F (1997) Plasma kinetic behavior in hyperlipidemic subjects of a lipidic microemulsion that binds to low density lipoprotein receptors. Lipids 32:627–633
Hirata RD, Hirata MH, Mesquita CH, Cesar TB, Maranhão RC (1999) Effects of apolipoprotein B-100 on the metabolism of a lipid microemulsion model rats. Biochim Biophys Acta 1437:53–62
Mahley RW (1988) Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240:622–630
Ruiz J, Kouiavskaia D, Migliorini M, Robinson S, Saenko EL, Gorlatova N, Li D, Lawrence D, Hyman BT, Weisgraber KH, Strickland DK (2005) The apo E isoform binding properties of the VLDL receptor reveal marked differences from LRP and the LDL receptor. J Lipid Res 46:1721–1731
Mangili OC, Moron Gagliardi AC, Mangili LC, Mesquita CH, Machado Cesar LA, Tanaka A, Schaefer EJ, Maranhão RC, Santos RD (2014) Favorable effects of ezetimibe alone or in association with simvastatin on the removal from plasma of chylomicrons in coronary heart disease subjects. Atherosclerosis 233:319–325
Maranhão RC, Garicochea B, Silva EL, Dorlhiac-Llacer P, Cadena SM, Coelho IJ, Meneghetti JC, Pileggi FJ, Chamone DA (1994) Plasma kinetics and biodistribution of a lipid emulsion resembling low-density lipoprotein in patients with acute leukemia. Cancer Res 54:4660–4666
Ho YK, Smith RG, Brown MS, Goldstein JL (1978) Low-density lipoprotein (LDL) receptor activity in human acute myelogenous leukemia cells. Blood 52:1099–1114
Maranhão RC, Garicochea B, Silva EL, Llacer PD, Pileggi FJ, Chamone DA (1992) Increased plasma removal of microemulsions resembling the lipid phase of low-density lipoproteins (LDL) in patients with acute myeloid leukemia: a possible new strategy for the treatment of the disease. Braz J Med Biol Res 25:1003–1007
Maranhão RC, Graziani SR, Yamaguchi N, Melo RF, Latrilha MC, Rodrigues DG, Couto RD, Schreier S, Buzaid AC (2002) Association of carmustine with a lipid emulsion: in vitro, in vivo and preliminary studies in cancer patients. Cancer Chemother Pharmacol 49:487–498
Hungria VT, Latrilha MC, Rodrigues DG, Bydlowski SP, Chiattone CS, Maranhão RC (2004) Metabolism of a cholesterol-rich microemulsion (LDE) in patients with multiple myeloma and a preliminary clinical study of LDE as a drug vehicle for the treatment of the disease. Cancer Chemother Pharmacol 53:51–60
Maranhão RC, Tavares ER, Padoveze AF, Valduga CJ, Rodrigues DG, Pereira MD (2008) Paclitaxel associated with cholesterol-rich nanoemulsions promotes atherosclerosis regression in the rabbit. Atherosclerosis 197:959–966
Tavares ER, Freitas FR, Diament J, Maranhão RC (2011) Reduction of atherosclerotic lesions in rabbits treated with etoposide associated with cholesterol-rich nanoemulsions. Int J Nanomed 6:2297–2304
Bulgarelli A, Leite AC Jr, Dias AA, Maranhão RC (2013) Anti-atherogenic effects of methotrexate carried by a lipid nanoemulsion that binds to LDL receptors in cholesterol-fed rabbits. Cardiovasc Drugs Ther 27:531–539
Leite AC Jr, Solano TV, Tavares ER, Maranhão RC (2015) Use of combined chemotherapy with etoposide and methotrexate, both associated to lipid nanoemulsions for atherosclerosis treatment in cholesterol-fed rabbits. Cardiovasc Drugs Ther 29:15–22
Daminelli EN, Martinelli AE, Bulgarelli A, Freitas FR, Maranhão RC (2016) Reduction of atherosclerotic lesions by the chemotherapeutic agent carmustine associated to lipid nanoparticles. Cardiovasc Drugs Ther 30:433–443
Lourenço-Filho DD, Maranhão RC, Méndez-Contreras CA, Tavares ER, Freitas FR, Stolf NA (2011) An artificial nanoemulsion carrying paclitaxel decreases the transplant heart vascular disease: a study in a rabbit graft model. J Thorac Cardiovasc Surg 141:1522–1528
Maranhão RC, Tavares ER (2015) Advances in non-invasive drug delivery for atherosclerotic heart disease. Expert Opin Drug Deliv 12:1135–1147
Maranhão RC, Vital CG, Tavoni TM, Graziani SR (2017) Clinical experience with drug delivery systems as tools to decrease the toxicity of anticancer chemotherapeutic agents. Expert Opin Drug Deliv 14:1217–1226
Ades A, Carvalho JP, Graziani SR, Amancio RF, Souen JS, Pinotti JA, Maranhão RC (2001) Uptake of a cholesterol-rich emulsion by neoplastic ovarian tissues. Gynecol Oncol 82:84–87
Azevedo CH, Carvalho JP, Valduga CJ, Maranhão RC (2005) Plasma kinetics and uptake by the tumor of a cholesterol-rich microemulsion (LDE) associated to etoposide oleate in patients with ovarian carcinoma. Gynecol Oncol 97:178–182
Graziani SR, Igreja FA, Hegg R, Meneghetti C, Brandizzi LI, Barboza R, Amancio RF, Pinotti JA, Maranhão RC (2002) Uptake of a cholesterol-rich emulsion by breast cancer. Gynecol Oncol 85:493–497
Stein Y, Halperin G, Stein O (1980) Biological stability of [3H]cholesteryl oleyl ether in cultured fibroblasts and intact rat. FEBS Lett 111:104–106
Rodrigues DG, Covolan CC, Coradi ST, Barboza R, Maranhão RC (2002) Use of a cholesterol-rich emulsion that binds to low-density lipoprotein receptors as a vehicle for paclitaxel. J Pharm Pharmacol 54:765–772
Valduga CJ, Fernandes DC, Lo Prete AC, Azevedo CH, Rodrigues DG, Maranhão RC (2003) Use of a cholesterol-rich microemulsion that binds to low-density lipoprotein receptors as vehicle for etoposide. J Pharm Pharmacol 55:1615–1622
Teixeira RS, Curi R, Maranhão RC (2004) Effects on Walker 256 tumour of carmustine associated with a cholesterol-rich microemulsion (LDE). J Pharm Pharmacol 56:909–914
Rodrigues DG, Maria DA, Fernandes DC, Valduga CJ, Couto RD, Ibañez OC, Maranhão RC (2005) Improvement of paclitaxel therapeutic index by derivatization and association to a cholesterol-rich microemulsion: in vitro and in vivo studies. Cancer Chemother Pharmacol 55:565–576
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Glass CK, Pittman RC, Keller GA, Steinberg D (1983) Tissue sites of degradation of apoprotein A-I in the rat. J Biol Chem 258:7161–7167
Chroni A, Liu T, Gorshkova I, Kan HY, Uehara Y, Von Eckardstein A, Zannis VI (2003) The central helices of ApoA-I can promote ATP-binding cassette transporter A1 (ABCA1)-mediated lipid efflux. Amino acid residues 220–231 of the wild-type ApoA-I are required for lipid efflux in vitro and high density lipoprotein formation in vivo. J Biol Chem 278:6719–6730
Laccotripe M, Makrides SC, Jonas A, Zannis VI (1997) The carboxyl-terminal hydrophobic residues of apolipoprotein A-I affect its rate of phospholipid binding and its association with high density lipoprotein. J Biol Chem 272:17511–17522
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Pitas RE, Innerarity TL, Mahley RW (1980) Cell surface receptor binding of phospholipid protein complexes containing different ratios of receptor-active and -inactive E apoprotein. J Biol Chem 255:5454–5460
Zannis VI, Just PW, Breslow JL (1981) Human apolipoprotein E isoprotein subclasses are genetically determined. Am J Hum Genet 33:11–24
Zannis VI, Breslow JL, Utermann G, Mahley RW, Weisgraber KH, Havel RJ, Goldstein JL, Brown MS, Schonfeld G, Hazzard WR, Blum C (1982) Proposed nomenclature of apoE isoproteins, apoE genotypes, and phenotypes. J Lipid Res 23:911–914
Kypreos KE, Zannis VI (2006) LDL receptor deficiency or apoE mutations prevent remnant clearance and induce hypertriglyceridemia in mice. J Lipid Res 47:521–529
Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J (1993) Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Investig 92:883–893
Segrest JP, Harvey SC, Zannis V (2000) Detailed molecular model of apolipoprotein A-I on the surface of high-density lipoproteins and its functional implications. Trends Cardiovasc Med 10:246–252
Brundert M, Ewert A, Heeren J, Behrendt B, Ramakrishnan R, Greten H, Merkel M, Rinninger F (2005) Scavenger receptor class B type I mediates the selective uptake of high-density lipoprotein-associated cholesteryl ester by the liver in mice. Arterioscler Thromb Vasc Biol 25:143–148
Zannis VI, Chroni A, Krieger M (2006) Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med (Berl) 84:276–294
Kent AP, Stylianou IM (2011) Scavenger receptor class B member 1 protein: hepatic regulation and its effects on lipids, reverse cholesterol transport, and atherosclerosis. Hepat Med 3:29–44
Acknowledgements
The authors thank Gayle Forbes (in memoriam) (Boston University) for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by National Council for Scientific and Technological Development (CNPq, Brasília, Brazil; Grant 473031/2012-4), São Paulo State Research Support Foundation (FAPESP, São Paulo, Brazil; Grant 14/03742-0), and Boston University (Boston, MA, USA). Dr. Maranhão has a Research Carrier Award from CNPq.
Conflict of interest
The authors declare they have no conflicts of interest.
About this article
Cite this article
Daminelli, E.N., Fotakis, P., Mesquita, C.H. et al. Tissue Uptake Mechanisms Involved in the Clearance of Non-Protein Nanoparticles that Mimic LDL Composition: A Study with Knockout and Transgenic Mice. Lipids 52, 991–998 (2017). https://doi.org/10.1007/s11745-017-4306-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-017-4306-6