Abstract
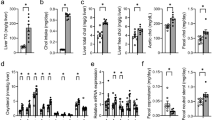
Mice were fed a control diet or a diet supplemented with hyodeoxycholic acid, the most abundant bile acid contained in pig bile, for 4 weeks, after which their serum and livers were collected. The contents of total fatty acids of serum and liver cholesteryl esters, and of liver triglycerides, were reduced following the administration of the hyodeoxycholic acid-supplemented diet, which was mainly due to the reductions in the contents of monounsaturated fatty acids. Free cholesterol contents in the serum and liver were not changed by hyodeoxycholic acid administration. Hyodeoxycholic acid administration reduced the gene expression levels of sterol regulatory element binding protein 1c, acetyl-CoA carboxylase, fatty acid synthase, and stearoyl-CoA desaturase-1. Hyodeoxycholic acid administration markedly changes the ratio of FXR-antagonist/FXR-agonist bile acids in the enterohepatic tissues of the mice (1.13 and 7.60 in hyodeoxycholic acid and control diet groups, respectively). Our findings demonstrate that hyodeoxycholic acid administration exerts the hypolipidemic effect in mice, in which downregulations of de novo lipogenesis and desaturation of saturated fatty acids are suggested to play important roles. In addition, regulation of FXR activation through the selective modification of the enterohepatic bile acid pool may be involved in the hypolipidemic effect of hyodeoxycholic acid administration.




Similar content being viewed by others
Abbreviations
- ACC:
-
Acetyl-CoA carboxylase
- CE:
-
Cholesteryl ester
- CYP7a1:
-
Cytochrome P450 7a1
- FXR:
-
Farnesoid X receptor
- FAS:
-
Fatty acid synthase
- HMGCAR:
-
3-Hydroxy-3-methylglutaryl coenzyme-A reductase
- LCMS:
-
Liquid chromatography–mass spectrometry
- MUFA:
-
Monounsaturated fatty acid
- PL:
-
Phospholipid
- SCD1:
-
Stearoyl-CoA desaturase-1
- SOAT2:
-
Sterol-O-acyltransferase-2
- SREBP1c:
-
Sterol regulatory element binding protein 1c
- TAG:
-
Triglyceride
References
Keitel V, Kubitz R, Häussinger D (2008) Endocrine and paracrine role of bile acids. World J Gastroenterol 14:5620–5629
Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B (2009) Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 89:147–191
Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J (2004) Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest 113:1408–1418
Bilz S, Samuel V, Morino K, Savage D, Choi CS, Shulman GI (2006) Activation of the farnesoid X receptor improves lipid metabolism in combined hyperlipidemic hamsters. Am J Physiol Endocrinol Metab 290:E716–E722
Einarsson C, Hillebrant CG, Axelson M (2001) Effects of treatment with deoxycholic acid and chenodeoxycholic acid on the hepatic synthesis of cholesterol and bile acids in healthy subjects. Hepatology 33:1189–1193
Song KH, Li T, Owsley E, Strom S, Chiang JY (2009) Bile acids activate fibroblast growth factor 19 signaling in human hepatocytes to inhibit cholesterol 7alpha-hydroxylase gene expression. Hepatology 49:297–305
Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ (2000) Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102:731–744
Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, Edwards PA (2006) Identification of novel pathways that control farnesoid X receptor-mediated hypocholesterolemia. Proc Natl Acad Sci USA 103:1006–1011
Zhang Y, Yin L, Anderson J, Ma H, Gonzalez FJ, Willson TM, Edwards PA (2010) Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. J Biol Chem 285:3035–3043
Fiorucci S, Cipriani F, Baldelli A, Mencarelli A (2010) Bile acid-activated receptors in the treatment of dyslipidemia and related disorders. Prog Lipid Res 49:171–185
Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, Lehmann JM (1999) Bile acids: natural ligands for an orphan nuclear receptor. Science 284(5418):1365–1368
Watanabe S, Tsuneyama K (2010) A triglyceride-lowering effect of cattle bile is associated with elevation of cholesterol levels and liver injury in mice. J Trad Med 27:179–185
Watanabe S, Tsuneyama K (2012) Cattle bile but not bear bile and pig bile induce lipid profile changes and fatty liver injury in mice. J Toxicol Sci 37:105–121
Watanabe S, Fujita K (2013) Pig bile reduces hepatic triglyceride content in mice. J Trad Med 30:190–197
Qiao X, Ye M, Pan DL, Miao WJ, Xiang C, Han J, Guo DA (2011) Differentiation of various traditional Chinese medicines derived from animal bile and gallstone: simultaneous determination of bile acids by liquid chromatography coupled with triple quadrupole mass spectrometry. J Chromatogr A 1218:107–117
Cohen-Solal C, Parquet M, Férézou J, Sérougne C, Lutton C (1995) Effects of hyodeoxycholic acid and alpha-hyocholic acid, two 6alpha-hydroxylated bile acids, on cholesterol and bile acid metabolism in the hamster. Biochim Biophys Acta 1257:189–197
Wang DQ, Tazuma S, Cohen DE, Carey MC (2003) Feeding natural hydrophilic bile acids inhibits intestinal cholesterol absorption: studies in the gallstone-susceptible mouse. Am J Physiol Gastrointest Liver Physiol 285:G494–G502
Hara A, Radin N (1978) Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 90:420–426
Alnouti Y, Csanaky IL, Klaassen CD (2008) Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J Chromatogr B 873:209–217
Russell DW, Setcehll KD (1992) Bile acid biosynthesis. Biochemistry 31:4737–4749
Li-Hawkins J, Gåfvels M, Olin M, Lund EG, Andersson U, Schuster G, Björkhem I, Russell DW, Eggertsen G (2002) Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J Clin Invest 110:1191–1200
Miyazaki M, Bruggink SM, Ntambi JM (2006) Identification of mouse palmitoyl-coenzyme A Delta9-desaturase. J Lipid Res 47:700–704
Miyazaki M, Kim YC, Ntambi JM (2001) A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J Lipid Res 42:1018–1024
Jonas A (1998) Regulation of lecithin cholesterol acyltransferase activity. Prog Lipid Res 37:209–234
Jonas A (2000) Lecithin cholesterol acyltransferase. Biochim Biophys Acta 1529:245–256
Bilz S, Samuel V, Morino K, Savage D, Choi CS, Shulman GI (2005) Activation of the farnesoid X receptor improves lipid metabolism in combined hyperlipidemic hamsters. Am J Physiol Endocrinol Metab 290:E716–E722
Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ (2002) Vitamin D receptor as an intestinal bile acid sensor. Science 296:1313–1316
Howard WR, Pospisil JA, Njolito E, Noonan DJ (2000) Catabolites of cholesterol synthesis pathways and forskolin as activators of farnesoid X-activated unclear receptor. Toxicol Appl Pharmacol 163:195–202
Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F (2013) Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 17:225–235
Hu X, Bonde Y, Eggertsen G, Rudling M (2014) Muricholic bile acids are potent regulators of bile acid synthesis via a positive feedback mechanism. J Intern Med 275:27–38
Cohen BI, Singhal AK, Mongelli J, Rothschild MA, McSherry CK, Mosbach EH (1983) Hydroxylation of secondary bile acids in the perfused prairie dog liver. Lipids 18:909–912
Bodin K, Lindbom U, Diczfalusy U (2005) Novel pathways of bile acid metabolism involving CYP3A4. Biochim Biophys Acta 1687:84–93
Alrefai WA, Gill RK (2007) Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res 24:1803–1823
Wang H, Chen J, Hollister K, Sowers LC, Forman BM (1999) Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 3:543–553
Okuyama H, Kobayashi T, Watanabe S (1996) Dietary fatty acids -the N-6/N-3 balance and chronic elderly diseases. Excess linoleic acid and relative N-3 deficiency syndrome seen in Japan. Prog Lipid Res 35:409–457
Jakobsson A, Westerberg R, Jacobsson A (2006) Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res 45:237–249
Smith WL (2005) Cyclooxygenases, peroxide tone and the allure of fish oil. Curr Opin Cell Biol 17:174–182
Serhan CN, Chiang N, Van Dyke TE (2008) Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 8:349–361
Ferré P, Foufelle F (2010) Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab Suppl 2:83–92
Acknowledgments
This work was supported in part by a Grant-in-Aid in Regional Innovation R&D Program from the Ministry of Economy, Trade and Industry, Japan and by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Research Project Number: 23590873). The authors wish to thank Mr. Shunsuke Komado and Mr. Atsushi Yoneyama (University of Toyama) for their help in analyzing gene expression and fatty acid composition.
Conflict of interest
S.W. received financial support from Kokando Co., Ltd., Japan. K.F. has no conflict of interest directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Watanabe, S., Fujita, K. Dietary Hyodeoxycholic Acid Exerts Hypolipidemic Effects by Reducing Farnesoid X Receptor Antagonist Bile Acids in Mouse Enterohepatic Tissues. Lipids 49, 963–973 (2014). https://doi.org/10.1007/s11745-014-3947-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-014-3947-y