Abstract
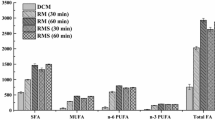
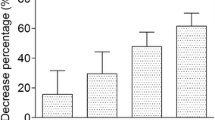
Several methods are available to extract total lipid and methylate fatty acids from a range of samples including red blood cells (RBC). Fatty acid analysis of human RBC can be undertaken using a two-step extraction and methylation or a combined one-step extraction and methylation procedure. The lipid composition of sheep RBC differs significantly from that of humans and may affect their extraction. The purpose of the current study was to examine the efficiency of extraction of lipid and detection of fatty acids from sheep RBC using a one-step procedure. Fatty acids were analysed using a one-step extraction and methylation procedure using methanol:toluene and acetyl chloride in comparison with a two-step procedure involving extraction of lipid using chloroform:methanol and separate methylation. Concentrations of saturated fatty acids including C16:0 and C18:0 were significantly higher (42.6 and 33.9 % respectively) following extraction using the one-step procedure compared with the two-step procedure. However, concentrations of some polyunsaturated fatty acids, including C20:5n-3 and C22:6n-3 were not significantly different between either procedure. The improved detection of fatty acids may be related to the ability of different solvents to extract different lipid fractions. The differential extraction of lipids and detection of fatty acids from sheep RBC may have important implications in studies examining the effect of dietary treatment on the possible health benefits of fatty acids.



Similar content being viewed by others
Abbreviations
- FAME:
-
Fatty acid methyl esters
- FID:
-
Flame ionisation detector
- LoD:
-
Limit of detection
- LCn-3PUFA:
-
Long-chain omega-3 polyunsaturated fatty acids
- LCn-6PUFA:
-
Long-chain omega-6 polyunsaturated fatty acids
- MUFA:
-
Monounsaturated fatty acids
- RBC:
-
Red blood cells
- SFA:
-
Saturated fatty acids
References
Brown AJ, Pang E, Roberts DC (1991) Persistent changes in the fatty acid composition of erythrocyte membranes after moderate intake of n-3 polyunsaturated fatty acids: study design implications. Am J Clin Nutr 54(4):668–673
Marckmann P, Lassen A, Haraldsdottir J, Sandstrom B (1995) Biomarkers of habitual fish intake in adipose tissue. Am J Clin Nutr 62(5):956–969
Clayton EH, Hanstock TL, Kable CJ, Hirneth SJ, Garg ML, Hazell PL (2008) Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids 43(11):1031–1038
Duckett SK, Wagner DG, Yates LD, Dolezal HG, May SG (1993) Effects of time on feed on beef nutrient composition. J Anim Sci 71(8):2079–2088
Scollan ND, Choi NJ, Kurt E, Fisher AV, Enser M, Wood JD (2001) Manipulating the fatty acid composition of muscle and adipose tissue in beef cattle. Br J Nutr 85(1):115–124
Christie WW (2003) Lipid analysis: isolation, separation, identification and structural analysis of lipids, 3rd edn. The Oily Press, Bridgwater
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226(1):497–509
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37(8):911–917
Hara A, Radin NS (1978) Lipid extraction of tissues with a low-toxicity solvent. Anal Biochem 90(1):420–426
Bocking C, Nockher WA, Schreiner M, Renz H, Pfefferle PI (2010) Development and validation of a combined method for the biomonitoring of omega-3/-6 fatty acids and conjugated linoleic acids in different matrices from human and nutritional sources. Clin Chem Lab Med 48(12):1757–1763
Lepage G, Roy CC (1986) Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res 27(1):114–120
Rodriguez-Palmero M, Lopez-Sabater MC, Castellote-Bargallo AI, De la Torre-Boronat MC, Rivero-Urgell M (1997) Comparison of two methods for the determination of fatty acid profiles in plasma and erythrocytes. J Chromatogr A 778(1–2):435–439
Ways P, Hanahan DJ (1964) Characterization and quantification of red cell lipids in normal man. J Lipid Res 5(3):318–328
Nelson GJ (1967) Lipid composition of erythrocytes in various mammalian species. BBA Lipid Lipid Met 144(2):221–232
NHMRC (2004) Australian code of practice for the care and use of animals for scientific purposes, 7th edn. Australian Government, Canberra
Iverson SJ, Lang SL, Cooper MH (2001) Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids 36(11):1283–1287
Or-Rashid MM, Fisher R, Karrow N, AlZahal O, McBride BW (2010) Fatty acid profile of colostrum and milk of ewes supplemented with fish meal and the subsequent plasma fatty acid status of their lambs. J Anim Sci 88(6):2092–2102
NATA (2009) Technical Note 17 - Guidelines for the validation and verification of chemical test methods. National Association of Testing Authorities Available online: http://www.nata.asn.au/phocadownload/publications/Technical_publications/Technotes_Infopapers/technical_note_17_apr09.pdf. Accessed 1 Dec 2011
Coakes SJ, Steed LG (2001) SPSS: analysis without anguish, version 10.0 for windows. Wiley, Sydney
SAS Institute Inc (1997) SAS/STAT Software: changes and enhancements through release 6.12. SAS Institute Inc, Cary
Littell RC, Henry PR, Ammerman CB (1998) Statistical analysis of repeated measures data using SAS procedures. J Anim Sci 76(4):1216–1231
Wang Z, Goonewardene LA (2004) The use of MIXED models in the analysis of animal experiments with repeated measures. Can J Anim Sci 84(1):1–11
Tabachnick BG, Fidell LS (2001) Using multivariate statistics, 4th edn. Allyn and Bacon, Sydney
Lepage G, Roy CC (1984) Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J Lipid Res 25(12):1391–1396
Simopoulos AP (1999) Essential fatty acids in health and chronic disease. Am J Clin Nutr 70(3 Suppl):S560–S569
Clayton EH, Hanstock TL, Garg ML, Hazell PL (2007) Long-chain omega-3 polyunsaturated fatty acids in the treatment of psychiatric illnesses in children and adolescents. Acta Neuropsych 19(2):92–103
Connellan JM, Masters CJ (1965) Fatty acid components of ovine erythrocyte lipids. Aust J Biol Sci 18:445–447
Hanahan DJ, Watts RM, Papajohn D (1960) Some chemical characteristics of the lipids of human and bovine erythrocytes and plasma. J Lipid Res 1(5):421–432
Nelson GJ (1967) Composition of neutral lipids from erythrocytes of common mammals. J Lipid Res 8(4):374–379
Rule DC (1997) Direct transesterification of total fatty acids of adipose tissue, and of freeze-dried muscle and liver with boron-trifluoride in methanol. Meat Sci 46(1):23–32
Araujo P, Nguyen TT, Froyland L, Wang JD, Kang JX (2008) Evaluation of a rapid method for the quantitative analysis of fatty acids in various matrices. J Chromatogr A 1212(1–2):106–113
Acknowledgments
We thank Greg Clark, Steven Huckell, Michael Loiterton, Rex Edis, Craig Rodham, John Wilkins, Susan Robertson, the Gulliver family, Jessica Rose, John Broster and Dr Stephanie Knott for technical assistance during the conduct of the study. We also thank Jamie Ayton for providing assistance with the gas chromatography. This study was conducted without dedicated external funding. This study was supported by in-kind provision of equipment by the Diagnostic and Analytical Systems division of NSW DPI.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Clayton, E.H., Gulliver, C.E., Piltz, J.W. et al. Improved Extraction of Saturated Fatty Acids but not Omega-3 Fatty Acids from Sheep Red Blood Cells Using a One-Step Extraction Procedure. Lipids 47, 719–727 (2012). https://doi.org/10.1007/s11745-012-3674-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-012-3674-1