Abstract
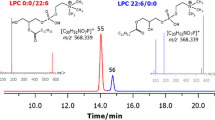
Liquid chromatography–electrospray tandem mass spectrometry (LC/ESI–MS/MS) was used to analyze phospholipids from three species of the anaerobic beer-spoilage bacterial genus Pectinatus. Analysis of total lipids by HILIC (Hydrophilic Interaction Liquid Chromatography) column succeeded in separating diacyl- and plasmalogen phospholipids. Plasmalogens were then analyzed by means of the ESI–MS/MS and more than 220 molecular species of four classes of plasmalogens (PlsCho (choline plasmalogen), PlsEtn (ethanolamine plasmalogen), PlsGro (glycerol plasmalogen), and PlsSer (serine plasmalogen)) were identified. Major molecular species were c-p19:0/15:0 PlsEtn and PlsSer, which accounted for more than 4% of the total lipids.





Similar content being viewed by others
Abbreviations
- CID:
-
Collision-induced dissociation
- DMSZ:
-
Deutsche Sammlung von Mikroorganismen und Zellkulturen (German Collection of Microorganisms and Cell Cultures)
- ECL:
-
Equivalent chain length
- FA:
-
Fatty acid(s)
- FAME:
-
Fatty acid methyl ester(s)
- GC-MS:
-
Gas chromatography–mass spectrometry
- HILIC:
-
Hydrophilic Interaction Liquid Chromatography
- LC/ESI–MS/MS:
-
Liquid chromatography–electrospray ionization tandem mass spectrometry
- PtdOH:
-
Phosphatidic acid
- PtdIns:
-
Phosphatidylinositol
- PlsCho:
-
Choline plasmalogen
- PlsEtn:
-
Ethanolamine plasmalogen
- PlsGro:
-
Glycerol plasmalogen
- PlsSer:
-
Serine plasmalogen
- RIBM:
-
Research Institute of Brewing and Malting
- RSD:
-
Relative standard deviation
- TLC:
-
Thin layer chromatography
References
Jespersen L, Jakobsen M (1996) Specific spoilage organisms in breweries and laboratory media for their detection. Food Microbiol 33:139–155
Sakamoto K, Konings WN (2003) Beer spoilage bacteria and hop resistance. Int J Food Microbiol 89:105–124
Basařová G, Šavel J, Basař P, Lejsek T (2010) Brewing, theory and practice of beer production (Pivovarství, teorie a praxe výroby piva). Vydavatelství VŠCHT Praha, Prague, pp 310–348
Helander IM, Haikara A (1995) Cellular fatty acyl and alkenyl residues in Megasphaera and Pectinatus species: contrasting profiles and detection of beer spoilage. Microbiology 141:1131–1137
Satokari R, Juvonen R, Mallison K, von Wright A, Haikara A (1998) Detection of beer spoilage bacteria Megasphaera and Pectinatus by polymerase chain reaction and colorimetric microplate hybridization. Int J Food Microbiol 45:119–127
Juvonen R, Koivula T, Haikara A (2008) Group-specific PCR-RFLP and real-time PCR methods for detection and tentative discrimination of strictly anaerobic beer-spoilage bacteria of the class Clostridia. Int J Food Microbiol 125:162–169
Schleifer KH, Leuteritz M, Weiss N, Ludwig W, Kirchhof G, Sedel-Rüfer H (1990) Taxonomic study of anaerobic, gram-negative, rod-shaped bacteria from breweries: Emended description of Pectinatus cerevisiiphilus and description of Pectinatus frisingensis sp. nov., Selenomonas lacticifex sp. nov., Zymophilus raffinosivorans gen. nov., sp. nov., and Zymophilus paucivorans sp. nov. Int J Syst Bacteriol 40:19–27
Matoulková D (2008) Strictly anaerobic bacteria in beer and in breweries. Kvasny Prum 54:338–343
Weber DG, Sahm K, Polen T, Wendisch VF, Antranikian G (2008) Oligonucleotide microarrays for the detection and identification of viable beer spoilage bacteria. J Appl Microbiol 105:951–962
Takatsuka Y, Kamio Y (2004) Molecular dissection of the Selenomonas ruminantium cell envelope and lysine decarboxylase involved in the biosynthesis of a polyamine covalently linked to the cell wall peptidoglycan layer. Biosci Biotechnol Biochem 68:1–19
Horrocks LA, Sharma M (1982) Plasmalogens and O-alkyl glycerophospholipids. In: Hawthorne JN, Ansell GB (eds) Phospholipids. Elsevier, Amsterdam, pp 51–93
Felde R, Spiteller G (1994) Search for plasmalogens in plants. Chem Phys Lipids 71:109–113
Mangold HK (1972) The search for alkoxylipids in plants. In: Snyder F (ed) Ether lipids: chemistry and biology. Academic Press, New York, pp 399–405
Goldfine H (2010) The appearance, disappearance and reappearance of plasmalogens in evolution. Prog Lipid Res 49:493–498
Christie WW, Han X (2010) Lipid analysis, 4th edn. The Oily Press, Bridgwater
Uran S, Larsen A, Jacobsen PB, Skotland T (2001) Analysis of phospholipid species in human blood using normal-phase liquid chromatography coupled with electrospray ionization ion-trap tandem mass spectrometry. J Chromatogr B 758:265–275
Olsson NU, Harding AJ, Harper C, Salem N (1996) High-performance liquid chromatography method with light scattering detection for measurements of lipid class composition: analysis of brains from alcoholics. J Chromatogr B 681:213–218
Mawatari S, Okuma Y, Fujino T (2007) Separation of intact plasmalogens and all other phospholipids by a single run of high-performance liquid chromatography. Anal Biochem 370:54–59
Nguyen HP, Schug KA (2008) The advantages of ESI-MS detection in conjunction with HILIC mode separations: fundamentals and applications. J Sep Sci 31:1465–1480
Schwalbe-Herrmann M, Willmann J, Leibfritz D (2010) Separation of phospholipid classes by hydrophilic interaction chromatography detected by electrospray ionization mass spectrometry. J Chromatogr A 1217:5179–5183
Kamleh A, Barrett MP, Wildridge D, Burchmore RJS, Scheltema RA, Watson DG (2008) Metabolomic profiling using Orbitrap Fourier transform mass spectrometry with hydrophilic interaction chromatography: a method with wide applicability to analysis of biomolecules. Rapid Commun Mass Spectrom 22:912–1918
Scherer M, Schmitz G, Liebisch G (2010) Simultaneous quantification of cardiolipin, bis(monoacylglycero)phosphate and their precursors by hydrophilic interaction LC-MS/MS including correction of isotopic overlap. Anal Chem 82:8794–8799
Hsu FF, Turk J (2009) Electrospray ionization with low-energy collisionally activated dissociation tandem mass spectrometry of glycerophospholipids: mechanisms of fragmentation and structural characterization. J Chromatogr B 877:2673–2695
Hsu FF, Turk J (2000) Characterization of phosphatidylethanolamine as a lithiated adduct by triple quadrupole, tandem mass spectrometry with electrospray ionization. J Mass Spectrom 35:596–606
Hsu FF, Turk J (2005) Studies on phosphatidylserine by tandem quadrupole and multiple stage quadrupole ion-trap mass spectrometry with electrospray ionization: Structural characterization and the fragmentation processes. J Am Soc Mass Spectr 16:1510–1522
Hsu FF, Turk J, Thukkani AK, Messner MC, Wildsmith KR, Ford DA (2003) Characterization of alkylacyl, alk-1-enylacyl and lyso subclasses of glycerophosphocholine by tandem quadrupole mass spectrometry with electrospray ionization. J Mass Spectrom 38:752–763
Hsu FF, Turk J (2005) Electrospray ionization with low-energy collisionally activated dissociation tandem mass spectrometry of complex lipids: structural characterization and mechanisms of fragmentation. In: Byrdwell WC (ed) Modern methods for lipid analysis by liquid chromatography/mass spectrometry and related techniques. AOCS Press, Champaign, pp 61–178
Lisa M, Holcapek M, Rezanka T, Kabatova N (2007) High-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry and gas chromatography-flame ionization detection characterization of delta 5-polyenoic fatty acids in triacylglycerols from conifer seed oils. J Chromatogr A 1146:67–77
Rezanka T, Mares P, Husek P, Podojil M (1986) Gas-chromatography mass-spectrometry and desorption chemical ionization mass-spectrometry of triacylglycerols from the green-alga Chlorella kessleri. J Chromatogr 355:265–271
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Kates M (1986) Techniques of lipidology: isolation, analysis and identification of lipids. In: Work TS, Work E (eds) Laboratory techniques in biochemistry and molecular biology, 2nd edn. Elsevier, Amsterdam, pp 220–223
Khuller GK, Goldfine H (1974) Phospholipids of Clostridium butyricum. V. Effects of growth temperature on fatty alk-1-enyl ether group, and phospholipid. J Lip Res 15:500–507
Nesbitt JA, Lennarz WJ (1965) Comparison of lipids and lipopolysaccharide from the bacillary and L forms of Proteus P18. J Bacteriol 89:1020–1025
Khuller GK, Goldfine H (1974) Phospholipids of Clostridium butyricum. V. Effects of growth temperature on fatty acid, 1-alkenyl ether group, and phospholipid composition. J Lipid Res 15:500–507
Christie WW (1989) Gas chromatography and lipids. A practical guide, Oily Press Ltd
Basconcillo LS, McCarry BE (2008) Comparison of three GC/MS methodologies for the analysis of fatty acids in Sinorhizobium meliloti: development of a micro-scale, one-vial method. J Chromatogr B 871:22–31
Moore LVH, Bourne DM, Moore WEC (1994) Comparative distribution and taxonomic value of cellular fatty acids in thirty-three genera of anaerobic Gram-negative bacilli. Int J Syst Bact 44:338–347
Gonzalez JM, Jurado V, Laiz L, Zimmermann J, Hermosin B, Saiz-Jimenez C (2004) Pectinatus portalensis nov sp., a relatively fast-growing, coccoidal, novel Pectinatus species isolated from a wastewater treatment plant. Antonie van Leeuwenhoek 86:241–248
Johnston NC, Goldfine H (1982) Effects of growth temperature on fatty acid and alk-1-enyl group compositions of Veillonella parvula and Megasphaera elsdenii phospholipids. J Bacteriol 149:567–575
Christie WW, Holman RT (1966) Mass spectrometry of lipids. I. Cyclopropane fatty acid esters. Lipids 1:176–182
Wood R, Reiser R (1965) Cyclopropane fatty acid metabolism; physical and chemical identification of propane ring metabolic products in the adipose tissue. J Am Oil Chem Soc 42:315–320
Harvey DJ (1984) Picolinyl derivatives for the characterization of cyclopropane fatty acids by mass spectrometry. Biomed Mass Spectrom 11:187–192
Hsu FF, Turk J (2006) Characterization of cardiolipin from Escherichia coli by electrospray ionization with multiple stage quadrupole ion-trap mass spectrometric analysis of [M–2H + Na]-ions. J Am Soc Mass Spectr 17:420–429
Mazzella N, Molinet J, Syakti AD, Barriol A, Dodi A, Bertrand JC, Doumenq P (2005) Effects of pure n-alkanes and crude oil on bacterial phospholipid classes and molecular species determined by electrospray ionization mass spectrometry. J Chromatogr B 822:40–53
de Vos P, Garrity G, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman WB (eds) (2009) Bergey’s manual of systematic bacteriology. Vol 3: The Firmicutes, Springer
Johnston NC, Baker JK, Goldfine H (2004) Phospholipids of Clostridium perfringens: a reexamination. FEMS Microbiol Lett 233:65–68
Mazzella N, Molinet J, Syakti AD, Dodi A, Doumenq P, Artaud J, Bertrand JC (2004) Bacterial phospholipid molecular species analysis by ion-pair reversed-phase HPLC/ESI/MS. J Lipid Res 45:1355–1363
Johnston NC, Aygun-Sunar S, Guan Z, Ribeiro AA, Daldal F, Raetz CRH, Goldfine H (2010) A phosphoethanolamine-modified glycosyl diradylglycerol in the polar lipids of Clostridium tetani. J Lipid Res 51:1953–1961
Richmond GS, Gibellini F, Young SA, Major L, Denton H, Lilley A, Smith TK (2010) Lipidomic analysis of bloodstream and procyclic form Trypanosoma brucei. Parasitology 137:1357–1392
Zemski Berry KA, Murphy RC (2004) Electrospray ionization tandem mass spectrometry of glycerophosphoethanolamine plasmalogen phospholipids. J Am Soc Mass Spectr 15:1499–1508
Holčapek M, Lísa M, Jandera P, Kabátová N (2005) Quantitation of triacylglycerols in plant oils using HPLC with APCI-MS, evaporative light-scattering, and UV detection. J Sep Sci 28:1315–1333 (this paper is cited in Supplementary material 1)
Rezanka T (1990) Identification of very long polyenoic acids as picolinyl esters by Ag+ ion-exchange high-performance liquid-chromatography, reversed-phase high-performance liquid-chromatography and gas-chromatography mass-spectrometry. J Chromatogr 513:344–348 (this paper is cited in Supplementary material 1)
Acknowledgments
This work was supported by the Ministry of Education, Youth and Sports of the Czech Republic (Project MSM2B08022 and MSM6046137305), Institutional Research Concept AV0Z50200510 and Research Centre 1M0570).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Řezanka, T., Siristova, L., Matoulková, D. et al. Hydrophilic Interaction Liquid Chromatography: ESI–MS/MS of Plasmalogen Phospholipids from Pectinatus Bacterium. Lipids 46, 765–780 (2011). https://doi.org/10.1007/s11745-011-3556-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-011-3556-y