Abstract
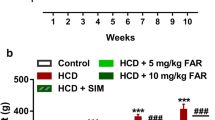
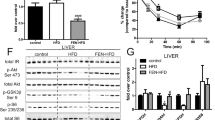
Obesity is associated with impaired fatty acid (FA) oxidation and increased de novo hepatic lipogenesis that may contribute to the development of hypertriglyceridemia, an important risk factor for the development of cardiovascular disease. Strategies to improve hepatocyte FA metabolism, including dietary interventions, are therefore important for the prevention of obesity-associated co-morbidities. Farnesol is consumed in the diet as a component of plant products. In the present study, we administered farnesol orally to rats for seven days and found significantly reduced serum triglyceride concentrations compared with controls. Potential mechanisms underlying the hypotriglyceridemic effect of farnesol were investigated using clone-9 cultured rat hepatocytes. Farnesol significantly upregulated expression of peroxisome proliferator-activated receptor alpha (PPARα) and the PPARα-regulated genes fatty acyl-CoA oxidase and carnitine palmitoyl transferase 1a, suggesting that increased hepatic FA oxidation may contribute to serum triglyceride lowering in rats. Farnesol did not change SREBP-1c mRNA levels, but significantly down-regulated fatty acid synthase (FAS) mRNA and protein levels and activity, indicating that attenuated lipogenesis may also contribute to hypotriglyceridemic effects of farnesol in vivo. Rescue experiments revealed that down-regulation of FAS by farnesol was not related to activation of PPARα, but rather was caused by a 9-cis retinoic acid mediated mechanism that involved down-regulation of retinoid X receptor β. Diets rich in plant products are associated with a lower risk of cardiovascular disease. Our findings suggest that farnesol may contribute to this protective effect by lowering serum TG levels.





Similar content being viewed by others
Abbreviations
- ACOX:
-
Fatty acyl-CoA oxidase
- CPT-1a:
-
Carnitine palmitoyl transferase 1a
- FA:
-
Fatty acid
- FAS:
-
Fatty acid synthase
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- PPARα:
-
Peroxisome-proliferator activated receptor alpha
- RXRα:
-
Retinoid X receptor alpha
- RXRβ:
-
Retinoid X receptor beta
- SREBP-1c:
-
Sterol regulatory element binding protein-1
- TG:
-
Triglycerides
References
Jacobson TA, Miller M, Schaefer EJ (2007) Hypertriglyceridemia and cardiovascular risk reduction. Clin Ther 29:763–777
Lewis GF (1997) Fatty acid regulation of very low density lipoprotein production. Curr Opin Lipidol 8:146–153
Marques-Lopes I, Ansorena D, Astiasaran I, Forga L, Martinez JA (2001) Postprandial de novo lipogenesis and metabolic changes induced by a high-carbohydrate, low-fat meal in lean and overweight men. Am J Clin Nutr 73:253–261
Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M (2002) Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol Endocrinol Metab 282:E46–E51
Elam MB, Wilcox HG, Cagen LM, Deng X, Raghow R, Kumar P, Heimberg M, Russell JC (2001) Increased hepatic VLDL secretion, lipogenesis, and SREBP-1 expression in the corpulent JCR:LA-cp rat. J Lipid Res 42:2039–2048
Svegliati-Baroni G, Candelaresi C, Saccomanno S, Ferretti G, Bachetti T, Marzioni M, De Minicis S, Nobili L, Salzano R, Omenetti A, Pacetti D, Sigmund S, Benedetti A, Casini A (2006) A model of insulin resistance and nonalcoholic steatohepatitis in rats: role of peroxisome proliferator-activated receptor-alpha and n-3 polyunsaturated fatty acid treatment on liver injury. Am J Pathol 169:846–860
Meigs TE, Simoni RD (1997) Farnesol as a regulator of HMG-CoA reductase degradation: characterization and role of farnesyl pyrophosphatase. Arch Biochem Biophys 345:1–9
He L, Mo H, Hadisusilo S, Qureshi AA, Elson CE (1997) Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J Nutr 127:668–674
Tatman D, Mo H (2002) Volatile isoprenoid constituents of fruits, vegetables and herbs cumulatively suppress the proliferation of murine B16 melanoma and human HL-60 leukemia cells. Cancer Lett 175:129–139
Horn TL, Long L, Cwik MJ, Morrissey RL, Kapetanovic IM, McCormick DL (2005) Modulation of hepatic and renal drug metabolizing enzyme activities in rats by subchronic administration of farnesol. Chemico Biol Interact 152:79–99
Ong TP, Heidor R, de Conti A, Dagli ML, Moreno FS (2006) Farnesol and geraniol chemopreventive activities during the initial phases of hepatocarcinogenesis involve similar actions on cell proliferation and DNA damage, but distinct actions on apoptosis, plasma cholesterol and HMGCoA reductase. Carcinogenesis 27:1194–1203
Meigs TE, Roseman DS, Simoni RD (1996) Regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase degradation by the nonsterol mevalonate metabolite farnesol in vivo. J Biol Chem 271:7916–7922
Voziyan PA, Goldner CM, Melnykovych G (1993) Farnesol inhibits phosphatidylcholine biosynthesis in cultured cells by decreasing cholinephosphotransferase activity. Biochem J 295(Pt 3):757–762
Murthy S, Tong H, Hohl RJ (2005) Regulation of fatty acid synthesis by farnesyl pyrophosphate. J Biol Chem 280:41793–41804
Hiyoshi H, Yanagimachi M, Ito M, Yasuda N, Okada T, Ikuta H, Shinmyo D, Tanaka K, Kurusu N, Yoshida I, Abe S, Saeki T, Tanaka H (2003) Squalene synthase inhibitors suppress triglyceride biosynthesis through the farnesol pathway in rat hepatocytes. J Lipid Res 44:128–135
Swinnen JV, Esquenet M, Goossens K, Heyns W, Verhoeven G (1997) Androgens stimulate fatty acid synthase in the human prostate cancer cell line LNCaP. Cancer Res 57:1086–1090
Keller RK, Zhao Z, Chambers C, Ness GC (1996) Farnesol is not the nonsterol regulator mediating degradation of HMG-CoA reductase in rat liver. Arch Biochem Biophys 328:324–330
Ide T, Tsunoda M, Mochizuki T, Murakami K (2004) Enhancement of insulin signaling through inhibition of tissue lipid accumulation by activation of peroxisome proliferator-activated receptor (PPAR) alpha in obese mice. Med Sci Monit 10:BR388–BR395
Kim H, Haluzik M, Asghar Z, Yau D, Joseph JW, Fernandez AM, Reitman ML, Yakar S, Stannard B, Heron-Milhavet L, Wheeler MB, LeRoith D (2003) Peroxisome proliferator-activated receptor-alpha agonist treatment in a transgenic model of type 2 diabetes reverses the lipotoxic state and improves glucose homeostasis. Diabetes 52:1770–1778
Giguere V (1999) Orphan nuclear receptors: from gene to function. Endocr Rev 20:689–725
Pineda Torra I, Jamshidi Y, Flavell DM, Fruchart JC, Staels B (2002) Characterization of the human PPARalpha promoter: identification of a functional nuclear receptor response element. Mol Endocrinol 16:1013–1028
Takahashi N, Kawada T, Goto T, Yamamoto T, Taimatsu A, Matsui N, Kimura K, Saito M, Hosokawa M, Miyashita K, Fushiki T (2002) Dual action of isoprenols from herbal medicines on both PPARgamma and PPARalpha in 3T3-L1 adipocytes and HepG2 hepatocytes. FEBS Lett 514:315–322
Hanley K, Komuves LG, Ng DC, Schoonjans K, He SS, Lau P, Bikle DD, Williams ML, Elias PM, Auwerx J, Feingold KR (2000) Farnesol stimulates differentiation in epidermal keratinocytes via PPARalpha. J Biol Chem 275:11484–11491
Lefebvre P, Chinetti G, Fruchart JC, Staels B (2006) Sorting out the roles of PPAR alpha in energy metabolism and vascular homeostasis. J Clin Invest 116:571–580
Cagen LM, Deng X, Wilcox HG, Park EA, Raghow R, Elam MB (2005) Insulin activates the rat sterol-regulatory-element-binding protein 1c (SREBP-1c) promoter through the combinatorial actions of SREBP, LXR, Sp-1 and NF-Y cis-acting elements. Biochem J 385:207–216
Wang Y, Jones Voy B, Urs S, Kim S, Soltani-Bejnood M, Quigley N, Heo YR, Standridge M, Andersen B, Dhar M, Joshi R, Wortman P, Taylor JW, Chun J, Leuze M, Claycombe K, Saxton AM, Moustaid-Moussa N (2004) The human fatty acid synthase gene and de novo lipogenesis are coordinately regulated in human adipose tissue. J Nutr 134:1032–1038
Amin D, Rutledge RZ, Needle SN, Galczenski HF, Neuenschwander K, Scotese AC, Maguire MP, Bush RC, Hele DJ, Bilder GE, Perrone MH (1997) RPR 107393, a potent squalene synthase inhibitor and orally effective cholesterol-lowering agent: comparison with inhibitors of HMG-CoA reductase. J Pharmacol Exp Ther 281:746–752
Hiyoshi H, Yanagimachi M, Ito M, Saeki T, Yoshida I, Okada T, Ikuta H, Shinmyo D, Tanaka K, Kurusu N, Tanaka H (2001) Squalene synthase inhibitors reduce plasma triglyceride through a low-density lipoprotein receptor-independent mechanism. Eur J Pharmacol 431:345–352
Scharnagl H, Schinker R, Gierens H, Nauck M, Wieland H, Marz W (2001) Effect of atorvastatin, simvastatin, and lovastatin on the metabolism of cholesterol and triacylglycerides in HepG2 cells. Biochem Pharmacol 62:1545–1555
Ugawa T, Kakuta H, Moritani H, Matsuda K, Ishihara T, Yamaguchi M, Naganuma S, Iizumi Y, Shikama H (2000) YM-53601, a novel squalene synthase inhibitor, reduces plasma cholesterol and triglyceride levels in several animal species. Br J Pharmacol 131:63–70
Bradfute DL, Silva CJ, Simoni RD (1992) Squalene synthase-deficient mutant of Chinese hamster ovary cells. J Biol Chem 267:18308–18314
Shimano H, Horton JD, Shimomura I, Hammer RE, Brown MS, Goldstein JL (1997) Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J Clin Invest 99:846–854
Sato R, Yang J, Wang X, Evans MJ, Ho YK, Goldstein JL, Brown MS (1994) Assignment of the membrane attachment, DNA binding, and transcriptional activation domains of sterol regulatory element-binding protein-1 (SREBP-1). J Biol Chem 269:17267–17273
Roder K, Zhang L, Schweizer M (2007) SREBP-1c mediates the retinoid-dependent increase in fatty acid synthase promoter activity in HepG2. FEBS Lett 581:2715–2720
Schweizer M, Roder K, Zhang L, Wolf SS (2002) Transcription factors acting on the promoter of the rat fatty acid synthase gene. Biochem Soc Trans 30:1070–1072
Key TJ, Fraser GE, Thorogood M, Appleby PN, Beral V, Reeves G, Burr ML, Chang-Claude J, Frentzel-Beyme R, Kuzma JW, Mann J, McPherson K (1999) Mortality in vegetarians and nonvegetarians: detailed findings from a collaborative analysis of 5 prospective studies. Am J Clin Nutr 70:516S–524S
Acknowledgments
This work was supported by a grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada. R.E.D. is a recipient of an NSERC postdoctoral fellowship. The authors would like to thank Chris Lange for assistance.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Duncan, R.E., Archer, M.C. Farnesol Decreases Serum Triglycerides in Rats: Identification of Mechanisms Including Up-Regulation of PPARα and Down-Regulation of Fatty Acid Synthase in Hepatocytes. Lipids 43, 619–627 (2008). https://doi.org/10.1007/s11745-008-3192-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-008-3192-3