Abstract
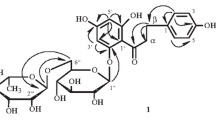
A mixture of five new ceramides was isolated from the aerial parts of Rantherium suaveolens and characterized by spectroscopic and chemical methods. Their structures were elucidated by spectroscopic and chemical methods as (2S, 3S, 4R, 2′R, 14E)-2-(2′-hydroxydocosanoylamino)-14-octadecene-1,3,4-triol (1), (2S,3S,4R,2′R, 14F)-2-(2′-hydroxytricosanoylamino)-14-octadecene-1,3,4-triol (2), (2S,3S,4R,2′R,14F)-2-(2′-hydroxytetracosanoylamino)-14-octadecene-1,3,4-triol (3), (2S,3S,4R,2′R,14E)-2-(2′-hydroxypentacosanoylamino)-14-octadecene-1,3,4-triol (4), and (2S,3S,4R,2′R,14E)-2-(2′-hydroxyhexacosanoylamino)-14-octadecene-1,3,4-triol (5).
Similar content being viewed by others
Abbreviations
- CC:
-
column chromatography
- HMBC:
-
heteronuclear multiple bond correlation
- LCB:
-
long-chain base
- MTPA:
-
α-methoxy-α-(trifluoromethyl)phenylacetyl acid
References
Alapetite, G.P. (1981) Flora of Tunisia, Tunisian Scientific Publications, Official Press, Republic of Tunisia, Tunis, Tunisia.
le Floc’h, E. (1983) Contribution to an Ethnobotanical Study of the Tunisian Flora, pp. 82–83, Tunisian Scientific Publications, Official Press, Republic of Tunisia, Tunis, Tunisia.
Issaoui, A., Kallala, A., Naffeti, M., and Akrimi, N. (1996) Plants from South Tunisia, Ministry of the Environment and Territorial Management, p. 174, State Secretariat of Scientific Research and Technology, Tunisia.
Oueslati, M.H., Ben Jannet, H., Abreu, P.J.M., and Mighri, Z. (2004) Alcools tetrahydropyraniques polyacetyleniques antibacteriens de la plate Rantherium suaveolens poussant dans le sud Tunisien, J. Soc. Alger. Chim. 14, 245–258.
Braham, H., Mighri, Z., Ben Jannet, H., Matthew, S., and Abreu, P.M. (2005) Antioxidant Phenolic Glycosides from Moricandia arvensis, J. Nat. Prod. 68, 517–522.
Oueslati, M.H., Ben Jannet, H., Abreu, P.J.M., and Mighri, Z. (2006) A New C9 nor-Isoprenoid Glucoside from Rantherium suaveolens, Nat. Prod. Res., in press.
Gaver, R.C., and Sweeley, C.C. (1965) Methods for Methanolysis of Sphingolipids and Direct Determination of Long-Chain Bases by Gas Chromatography, J. Am. Oil Chem. Soc. 42, 294–298.
Vogel, A. (1978) Vogel’s Textbook of Practical Organic Chemistry, 4th edn., pp. 292, Longman, London.
McLaughlin, J.L. (1991) Crown Gall Tumours on Potato Discs and Brine Shrimp Lethality: Two Simple Bioassays for Higher Plant Screening and Fractionation, in Methods in Plant Biochemistry (Hostettman, K., eds.), Vol. 6, pp. 1–32, Academic, London.
Krishna, N., Muralidhar, P., Kumar, M.M., Rao, D.V., and Rao, C.B. (2004) New Sphingosines from a Gorgonian, Pseudopterogorgia australiensis Ridley, of the Indian Ocean, J. Nat. Prod. 67, 1423–1425.
Zhan, Z.-J., and Yue, J.-M. (2003) New Glyceroglycolipid and Ceramide from Premna microphylla, Lipids 38, 1299–1303.
Qi, S.-H., Wu, D.-G., Zhang, S., and Luo, X.-D. (2003) A New Tetranortriterpenoid from Dysoxylum lenticellatum, Z. Naturforsch. 58b, 1128–1132.
Gao, J.-M., Dong, Z.-J., and Liu, J.-K. (2001) A New Ceramide from the Basidiomycete Russula cyanoxantha, Lipids 36, 175–180.
Loukaci, A., Bultel-Poncé, V., Longeon, A., and Guyot, M. (2000) New Lipids from the Tunicate Cystodytes cf. dellechiajei, as PLA2 Inhibitors, J. Nat. Prod. 63, 799–802.
Asai, N., Fusetani, N., Matsunaga, S., and Sasaki, J. (2000) Sex Pheromones of the Hair Crab Erimacrus isenbeckii. Part 1: Isolation and Structures of Novel Ceramides, Tetrahedron 56, 9895–9899.
Rubino, F.M., Zecca, L., and Sonnino, S. (1994) Characterization of Complex Mixture of Ceramides by Fast Atom Bombardment and Precursor and Tandem Mass Spectrometry, in Mass Spectrometry, Biol. Mass Spectrom. 23, 82–90.
Rossi, R., and Veracini, C.A. (1982) Insect Pheromone Components. Use of 13C NMR Spectroscopy for Assigning the Configuration of C=C Double Bonds of Monoenic or Dienic Pheromone Components and for Quantitative Determination of Z/E Mixtures, Tetrahedron 38, 629–644.
Cateni, F., Zilic, J., Falsone, G., Scialino, G., and Banfi, E. (2003) New Cerebrosides from Euphorbia peplis, L.: Antimicrobial Activity Evaluation, Bioorg. Med. Chem. Lett. 15, 4345–4350.
Dale, J.A., and Mosher, H.S. (1973) Nuclear Magnetic Resonance Enantiomer Reagents. Configurational Correlations via Nuclear Magnetic Resonance Chemical Shifts of Diastereomic Mandelate, O-Methylmandelate, and α-Methoxy-α-trifluoromethylphenylacetate (MTPA) Esters, J. Am. Chem. Soc. 95, 512–519.
Rasmussen, H.B., Christensen, S.B., Kvist, L.P., Kharazmi, A., and Huansi, A.G. (2000) Absolute Configuration and Antiprotozoal Activity of Minquartynoic Acid, J. Nat. Prod. 63, 1295–1296.
Seco, J.M., Quiñoá, E., and Riguera, R. (2001) A Practical Guide for the Assignment of the Absolute Configuration of Alcohols, Amines and Carboxylic Acids by NMR, Tetrahedron: Asymmetry 12, 2915–2925.
Su, B.-N., Misico, R., Park, E.J., Santarsiero, B.D., Mesecar, A.D., Fong, H.H.S., Pezzuto, J.M., and Kinghorn, A.D. (2002) Isolation and Characterization of Bioactive Principles of the Leaves and Stems of Physalis philadelphica, Tetrahedron 58, 3453–3466.
Liu, J.-K., Hu, L., and Dong, Z.-J. (2003) A Glucosylceramide with a Novel Ceramide and Three Ceramides from the Basidiomycete Cortinarius umidicola, Lipids 38, 669–675.
De Vivar, M.E.D., Seldes, A.M., and Maier, M.S. (2002) Two Novel Glucosylceramides from Gonads and Body Walls of the Patagonian Starfish Allostichaster inaequalis, Lipids 37, 597–603.
Costantino, V., Fattorusso, E., Mangoni, A., Di Rosa, M., Ianaro, A., and Maffia, A. (1996) Glycolipids from Sponges. IV. Immunomodulating Glycosyl Ceramides from the Marine Sponge Agelas dispar, Tetrahedron 52, 1573–1578.
Li, H.-y., Matsunaga, S., and Fusetani, N. (1995) Halicylindrosides, Antifungal and Cytotoxic Cerebrosides from the Marine Sponge Halichondria cylindrata, Tetrahedron 51, 2273–2280.
Jin, W., Rinehart, K.L., and Jares-Erijman, E. (1994) Ophidiacerebrosides: Cytotoxic Glycosphingolipids Containing a Novel Sphingosine from a Sea Star, J. Org. Chem. 59, 144–147.
Iguchi, R., Natori, T., and Komori, T. (1990) Isolation and Characterization of Acanthacerebroside B and Structure Elucidation of Related, Nearly Homogenous Cerebrosides, Liebigs Ann. Chem., 51–55.
Sugiyama, S., Honda, M., and Komori, T. (1988) Asymmetric Synthesis of Phytosphingosine and Phytosphingosine Anhydro Base: Assignment of the Absolute Stereochemistry. Liebigs Ann. Chem., 619–625.
Sugiyama, S., Honda, M., Higuchi, R., and Komori, T. (1991) Stereochemistry of the Four Diastereomers of Ceramide and Ceramide Lactoside, Liebigs Ann. Chem. 349–356.
Murakami, T., and Taguchi, K. (1999) Stereocontrolled Synthesis of Novel Phytosphingosine-type Glucosaminocerebrosides, Tetrahedron 55, 989–1004.
Cremesti, A.E., and Fischi, A.S. (2000) Current Methods for the Identification and Quantitation of Ceramides: An Overview, Lipids 35, 937–945.
Kester, M., and Kolesnick, R. (2003) Sphingolipids as Therapeutics. Pharmacol. Res. 47, 365–371.
Sot, J., Goñi, F. M., and Alonso, A. (2005) Molecular Associations and Surface-Active Properties of Short- and Long-N-Acyl Chain Ceramides, Biochim. Biophys. Acta 1711, 12–19.
Stoica, B.A., Movsesyan, V.A., Knoblach, S.M., and Faden, A.I. (2005) Ceramide Induces Neuronal Apoptosis Through Mitogen-Activated Protein Kinases and Causes Release of Multiple Mitochondrial Proteins, Mol. Cell. Neurosci. 29, 355–371.
Azuma, H., Takao, R., Niiro, H., Shikata, K., Tamagaki, S., Tachibana, T., and Ogino, K. (2003) Total Synthesis of Symbioramide Derivatives from l-Serine and Their Antileukemic Activities, J. Org. Chem. 68, 2790–2797.
Aouali, N., Eddabra, L., Macadré, J., and Morjani, H. (2005) Immunosuppressors and Reversion of Multidrug-Resistance, Crit. Rev. Oncol. Hematol., 56, 61–70.
Sperling, P., and Heinz, E. (2003) Plant Sphingolipids: Structural Diversity, Biosynthesis, First Genes and Functions, Biochim. Biophys. Acta 1632, 1–15.
Jiang, H., Huang, X., Nakanishi, K., and Berova, N. (1999) Nanogram Scale Absolute Configurational Assigment of Ceramides by Circular Dichroism, Tetrahedron Lett. 40, 7645–7649.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Oueslati, M.H., Mighri, Z., Jannet, H.B. et al. New ceramides from Rantherium suaveolens . Lipids 40, 1075–1079 (2005). https://doi.org/10.1007/s11745-005-1472-3
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-005-1472-3