Abstract
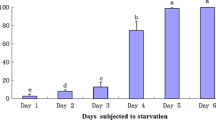
Daphnia magna is a common crustacean that is adapted to brief speels of fasting. Lipids are naturally a major component of their diet and are stored as energy reserves. However, there has been some controversy in the literature on the extent to which dietary lipids are used directly for complex lipid formation in Daphnia. We examined lipid metabolism in D. magna by labeling the animals using [1-14C]acetate and then followed the turnover of radiolabeled lipids during a pulse chase. Daphnia were either fed or maintained without food during the chase period. The decrease in radioactivity during the chase was relatively unaffected by feeding, although there were some differences in the distribution of radioactivity between lipid classes or individual FA. The polar lipids, which were four times better labeled than nonpolar lipids, contained the most radioactivity in the zwitterionic phosphoglycerides, PE and PC. Under the experimental conditions, the turnover of the polar membrane lipids was unaffected by feeding. Within nonpolar lipids, TAG accounted for up to about 80% of the label, followed by DAG. Overall, our data show that D. magna is capable of high rates of lipid radiolabeling de novo and, in addition, is able to use—and indeed may be dependent on—some dietary components such as the PUFA linoleate and α-linolenate. The results also clearly show that Daphnia is able to tolerate brief spells of fasting (24 h) with very little change to its lipid metabolism.
Similar content being viewed by others
Abbreviations
- DMOX:
-
4,4-dimethyloxazoline
- dpm:
-
disintegration per minute
- PG:
-
phosphatidylglycerol
- PL:
-
polar lipids
References
Nakamura, M.T., and Nara, T.Y. (2003) Essential Fatty Acid Synthesis and Its Regulation in Mammals, Prostaglandins, Leukotr. Essent. Fatty Acids 68, 145–150.
Lauritzen, L., and Hansen, H.S. (2003) Which of the n−3 FA Should Be Called Essential? Lipids 38, 889–891.
Martin-Creuzburg, D., and Von Elert, E. (2004) Impact of 10 Dietary Sterols on Growth and Reproduction of Daphnia galeata, J. Chem. Ecol. 30, 483–500.
Teshima, S., Kanazava, W.A., and Koshio, S. (1993) Recent Developments in Nutrition and Microparticulate Diets of Larval Prawns, Isr. J. Aquacult.-Bamidgen 45, 175–184.
Teshima, S., and Kanazava, W.A. (1986) Nutritive Value of Sterols for the Juvenile Prawn, Bull. Jpn. Soc. Sci. Fish. 52, 1417–1422.
Kanazava, A. (2001) Sterols in Marine Invertebrates, Fish. Sci. 67, 997–1007.
Ackman, R.G. (1999) Comparison of Lipids in Marine and Freshwater Organisms, in Lipids in Freshwater Ecosystems (Arts, M.T., and Wainman, B.C., eds.), pp. 262–298, Springer, New York.
Arts, M.T., Evans, M.S., and Robarts, R.D. (1992) Seasonal Patterns of Total and Energy Reserve Lipids of Dominant Zooplanktonic Crustaceans from a Hyper-eutrophic Lake, Oecologia 90, 560–571.
Falk-Petersen, S., Sargent, J.R., Lonne, O.J., and Timofeev, S. (1999) Functional Biodiversity of Lipids in Antarctic Zooplankton: Calanoides acutus, Calanus propinquus, Thysanoessa macrura and Euphausia crystallorophias, Polar Biol. 21, 37–47.
Olsen, Y. (1999) Lipids and Essential Fatty Acids in Aquatic Food Webs: What Can Freshwater Ecologists Learn from Mariculture? in Lipids in Freshwater Ecosystems (Arts, M.T., and Wainman, B.C., eds.), pp. 161–203, Springer, New York.
Hagen, W. (1999) Reproductive Strategies and Energetic Adaptations of Polar Zooplankton, Invertebr. Reprod. Dev. 36, 25–34.
Hagen, W., and Auel, H. (2001) Seasonal Adaptations and the Role of Lipids in Oceanic Zooplankton, Zool.-Anal. Complex Syst. 104, 313–326.
Arts, M.T. (1999) Lipids in Freshwater Zooplankton: Selected Ecological and Physiological Aspects, in Lipids in Freshwater Ecosystems (Arts, M.T., and Wainman, B.C., eds.), pp. 71–90, Springer, New York.
Goulden, C.E., Moeller, R.E., McNair, J.N., and Place, A.R. (1999) Lipid Dietary Dependencies in Zooplankton, in Lipids in Freshwater Ecosystems (Arts, M.T., and Wainman, B.C., eds.), pp. 91–109, Springer, New York.
Goulden, C.E., and Place, A.R. (1990) Fatty Acid Synthesis and Accumulation Rates in Daphniids, J. Exp. Zool. 256, 168–178.
Goulden, C.E., and Place, A.R. (1993) Lipid Accumulation and Allocation in Daphniid Cladocera, Bull. Mar. Sci. 53, 106–114.
Farkas, T., Kariko, K., and Csengeri, I. (1981) Incorporation of [I-14C]Acetate into Fatty Acids of the Crustaceans Daphnia magna and Cyclops strenus in Relation to Temperature, Lipids 16, 418–422.
Arts, M.T., Robarts, R.D., and Evans, M.S. (1993) Energy Reserve Lipids of Zooplanktonic Crustaceans from an Oligotrophic Saline Lake in Relation to Food Resources and Temperature, Can. J. Fish. Aquat. Sci. 50, 2404–2420.
Wainman, B.C., McQueen, D.J., and Lean, D.R.S. (1993) Seasonal Trends in Zooplankton Lipid Concentration and Class in Freshwater Lakes, J. Plankton Res. 15, 1319–1332.
Cauchie, H.M., Jasper-Versali, M.F., Hoffmann, L., and Thorne, J.P. (1999) Analysis of the Seasonal Variation in Biochemical Composition of Daphnia magna Straus (Crustacea: Branchiopoda: Anomopoda) from an Acrated Wastewater Stabilisation Pond, Ann. Limnol./Intl. J. Limnol. 35, 223–231.
Sargent, J.R., and Henderson, R.J. (1986) Lipids, in The Biological Chemistry of Marine Copepods (Corner, E.D.S., and O'Hara, S.C.M., eds.), pp. 59–108, Clarendon Press, Oxford.
Wakil, S.J., Stoops, J.K., and Joshi, V.C. (1983) Fatty Acid Synthesis and Its Regulation, Annu. Rev. Biochem. 52, 537–579.
Gurr, M.I., Harwood, J.L., and Frayn, K.N. (2002) Lipid Biochemistry, 5th edn., Blackwells, Oxford.
Knops, M., Altenburger, R., and Segner, H. (2001) Alterations of Physiological Energetics, Growth and Reproduction of Daphnia magna Under Toxicant Stress, Aquat. Toxicol. 53, 79–90.
Sakai, M. (2001) Chronic Toxicity Test with Daphnia magna for Examination of River Water Quality, Food Contam. Agric. Wastes 36, 67–74.
Barata, C., Carlos-Navarro, J., Varo, I., Carmen Riva, M., Arun, S., and Porte, C. (2005) Changes in Antioxidant Enzyme Activities, Fatty Acid Composition and Lipid Peroxidation in Daphnia magna During the Aging Process, Compar. Biochem. Physiol. B: Biochem. Mol. Biol. 140B, 81–90.
Ballantyne, A.P., Brett, M.T., and Schindler, D.E. (2003) The Importance of Dietary Phosphorus and Highly Unsaturated Fatty Acids for Sockeye (Oncorhynchus nerka) Growth in Lake Washington—A Bioenergetics Approach, Can. J. Fish. Aquat. Sci. 60, 12–22.
Von Elert, E. (2004) Food Quality Constraints in Daphnia: Interspecific Differences in the Response to the Absence of a Long Chain Polyunsaturated Fatty Acid in the Food Source, Hydrobiologia 526, 187–196.
Becker, C., Feuchtmayr, H., Brepohl, D., Santer, B., and Boersma, M. (2004) Differential Impacts of Copepods and Cladocerans on Lake Seston, and Resulting Effects on Zooplankton Growth, Hydrobiologia 526, 197–207.
Park, S., Müller-Navarra, D., and Goldman, C.R. (2003) Seston Essential Fatty Acids and Carbon to Phosphorus Ratios as Predictors for Daphnia pulex Dynamics in a Large Reservoir, Lake Berryessa, Hydrobiologia 505, 171–178.
Ravet, J.L., Brett, M.T., and Müller-Navarra, D.C. (2003) A Test of the Role of Polyunsaturated Fatty Acids in Phytoplankton Food Quality for Daphnia Using Liposome Supplementation, Limnol. Oceanogr. 48, 1938–1947.
Von Elert, E. (2002) Determination of Limiting Polyunsaturated Fatty Acids in Daphnia galeata Using a New Method to Enrich Food Algae with Single Fatty Acids, Limnol. Oceanogr. 47, 1764–1773.
Sundbom, M., and Vrede, T. (1997) Effects of Fatty Acid and Phosphorus Content of Food on the Growth, Survival and Reproduction of Daphnia, Freshwater Biol. 38, 665–674.
Kainz, M., and Mazumder, A. (2005) Effects of Algal and Bacterial Diet on Methyl Mercury Concentrations in Zooplankton, Environ. Sci. Technol. 39, 1666–1672.
Wacker, A., and Von Elert, E. (2001) Polyunsaturated Fatty Acids: Evidence for Non-substitutable Biochemical Resources in Daphnia galeata, Ecology 82, 2507–2520.
Ahmadjian, V. (1967) The Lichen Symbiosis, Blaisdell, Waltham, MA.
Kainz, M., Lucotte, M., and Parrish, C.C. (2002) Methyl Mercury in Zooplankton—The Role of Size, Habitat and Food Quality, Can. J. Fish. Aquat. Sci. 59, 1606–1615.
Folch, J., Lees, M., and Sloane Stanley, G.H. (1957) A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues, J. Biol. Chem. 226, 497–509.
Garbus, J., De Luca, H.F., Loomans, M.E., and Strong, F.M. (1963) The Rapid Incorporation of Phosphate into Mitochondrial Lipids, J. Biol. Chem. 238, 59–63.
Kates, M. (1986) Techniques in Lipidology, 2nd edn., Elsevier, Amsterdam.
Elendt, B.P. (1989) Effects of Starvation on Growth, Reproduction, Survival and Biochemical Composition of Daphnia magna, Arch. Hydrobiol. 116, 415–433.
Vrede, T., Persson, J., and Aronsen, G. (2002) The Influence of Food Quality (P:C Ratio) and Somatic Growth Rate of Daphnia, Limnol. Oceanogr. 47, 487–494.
Farkas, T., Nemecz, G.Y., and Csengeri, I. (1984) Differential Response of Lipid Metabolism and Membrane Physical State by an Actively and Passively Overwintering Planktonic Crustacean, Lipids 19, 436–442.
Bychek, E.A., and Gushchina, I.A. (1999) Age-Dependent Changes of Lipid Composition in Daphnia magna, Biochemistry-Moscow 64, 543–545.
Cripps, C., Blomquist, G.J., and Renobales, D.M. (1986) De novo Biosynthesis of Linoleic Acid in Insects, Biochim. Biophys. Acta 876, 572–580.
Blomquist, G.J., Borgeson, C.E., and Vundla, M. (1991) Polyunsaturated Fatty Acids and Eicosanoids in Insects, Insect Biochem. 21, 99–106.
Weinert, J., Blomquist, G.J., and Borgeson, C.E. (1993) De novo Biosynthesis of Linoleic Acid in 2 Non-insect Invertebrates—The Land Slug and the Garden Snail, Experientia 49, 919–921.
Avery, S.V., Lloyd, D., and Harwood, J.L. (1994) Changes in Membrane Fatty Acid Composition and Δ-12-Desaturase Activity During Growth of Acanthamoeba castellanii in Batch Culture, J. Eukaryot. Microbiol. 41, 396–401.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Bychek, E.A., Dobson, G.A., Harwood, J.L. et al. Daphnia magna can tolerate short-term starvation without major changes in lipid metabolism. Lipids 40, 599–608 (2005). https://doi.org/10.1007/s11745-005-1421-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-005-1421-1