Abstract
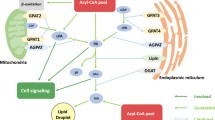
Glycerol kinase (ATP:glycerol-3-phosphotransferase, EC 2.7.1.30, glycerokinase) (Gyk) has a central role in plasma glycerol extraction and utilization by tissues for lipid biosynthesis. Gyk deficiency causes various phenotypic changes ranging from asymptomatic hyperglycerolemia to a severe metabolic disorder with growth and psychomotor retardation. To better understand the potential role of Gyk in tissue lipid metabolism, we determined phospholipid (PL), cholesterol (Chol), and triacylglycerol (TG) mass in a number of tissues from mice lacking Gyk. We report a tissue-dependent response to Gyk gene deletion. Tissues with elevated total PL mass (brain, kidney, muscle) were characterized by the increased mass of ethanolamine glycerophospholipids (EtnGpl), choline glycerophospholipids, and phosphatidylserine (PtdSer). In heart, lipid changes were characterized by a reduction in total PL, including decreased EtnGpl, phosphatidylinositol, and PtdSer mass and decreased TG and FFA mass. In parallel with tissue PL alterations, tissue Chol was also changed, maintaining a normal Chol/PL ratio. Under conditions of Gyk deficiency, we speculate that glycerol-3-phosphate and lipid production is maintained via alternative biosynthesis, including glycolysis, glyceroneogenesis, or by direct acylation of glycerol in brain, muscle, kidney, and liver, but not in heart.
Similar content being viewed by others
Abbreviations
- Chol:
-
cholesterol
- CerPCho:
-
sphingomyelin
- ChoGpl:
-
choline glycerophospholipids
- Gyk:
-
glycerol kinase
- Gro-3-P:
-
glycerol-3-phosphate
- EtnGpl:
-
ethanolamine glycerophospholipids
- PL:
-
phospholipids
- PtdSer:
-
phosphatidylserine
- PtdIns:
-
phosphatidylinositol
- TG:
-
triacylglycerol.
References
Reshef, L., Olswang, Y., Cassuto, H., Blum, B., Croniger, C.M., Kalhan, S.C., Tilghman, S.M., and Hanson, R.W. (2003) Glyceroneogenesis and the Triglyceride/Fatty Acid Cycle, J. Biol. Chem. 278, 30413–30416.
Stroud, R.M., Savage, D., Miercke, L.J.W., Lee, J.K., Khademi, S., and Harries, W. (2003) Selectivity and Conductance Among the Glycerol and Water Conducting Aquaporine Family of Channels, FEBS Lett. 555, 79–84.
Bortz, W.M., Paul, P., Haff, A.C., and Holmes, W.L. (1972) Glycerol Turnover and Oxidation in Man, J. Clin. Invest. 51, 1537–1546.
Baba, H., Zhang, X.J., and Wolfe, R.R. (1995) Glycerol Gluconeogenesis in Fasting Humans, Nutrition 11, 149–153.
Agteresch, H.J., Leij-Halfwerk, S., Van Den Berg, J.W., Hordijk-Luijk, C.H., Wilson, J.H., and Dagnelie, P.C. (2000) Effects of ATP Infusion on Glucose Turnover and Gluconeogenesis in Patients with Advanced Non-small-cell Lung Cancer, Clin. Sci. (London) 98, 689–695.
Previs, S.F., Martin, S.K., Hazey, J.W., Soloviev, M.V., Keating, A.P., Lucas, D., David, F., Koshy, J., Kirschenbaum, D.W., Tserng, K.Y., and Brunengraber, H. (1996) Contributions of Liver and Kidneys to Glycerol Production and Utilization in the Dog, Am. J. Physiol. 271, E1118-E1124.
Guo, Z., and Jensen, M.D. (1999) Blood Glycerol Is an Important Precursor for Intramuscular Triacylglycerol Synthesis, J. Biol. Chem. 274, 23702–23706.
Nguyen, N.H., Brathe, A., and Hassel, B. (2003) Neuronal Uptake and Metabolism of Glycerol and the Neuronal Expression of Mitochondrial Glycerol-3-phosphate Dehydrogenase, J. Neurochem. 85, 831–842.
Pascual de Bazan, H.E., and Bazan, N.G. (1976) Phospholipid Composition and [14C]Glycerol Incorporation into Glycerolipids of Toad Retina and Brain, J. Neurochem. 27, 1051–1057.
Robinson, J., and Newsholme, E.A. (1967) Glycerol Kinase Activities in Rat Heart and Adipose Tissue, Biochem. J. 104, 2C-4C.
Wolfe, R.R., Klein, S., Carraro, F., and Weber, J.M. (1990) Role of Triglyceride-Fatty Acid Cycle in Controlling Fat Metabolism in Humans During and After Exercise, Am. J. Physiol. 258, E382-E389.
Carmaniu, S., and Herrera, E. (1980) Comparative Utilization in vivo of [U-14C]Glycerol, [2-3H]Glycerol, [U-14C]Glucose and [1-14C]Palmitate in the Rat, Arch. Int. Physiol. Biochim. 88, 255–263.
Glaumann, H., Bergstrand, A., and Ericsson, J.L. (1975) Studies on the Synthesis and Intracellular Transport of Lipoprotein Particles in Rat Liver, J. Cell Biol. 64, 356–377.
Abdel-Latif, A.A., and Smith, J.P. (1970) In vivo Incorporation of Choline, Glycerol and Orthophosphate into Lecithin and Other Phospholipids of Subcellular Fractions of Rat Cerebrum, Biochim. Biophys. Acta 218 134–140.
Lapetina, E.G., Rodriguez de Lores Arnaiz, G., and De Robertis, E. (1969) Turnover Rates for Glycerol, Acetate and Orthophosphate in Phospholipids of the Rat Cerebral Cortex, Biochim. Biophys. Acta 176, 643–646.
Peroni, O., Large, V., Odeon, M., and Beylot, M. (1996) Measuring Glycerol Turnover, Gluconeogenesis from Glycerol, and Total Gluconeogenesis with [2–13C] Glycerol: Role of the Infusion-Sampling Mode, Metabolism 45 897–901.
Lumeng, L., Bremer, J., and Davis, E.J. (1976) Suppression of the Mitochondrial Oxidation of (−)-Palmitylcarnitine by the Malate-Aspartate and α-Glycerophosphate Shuttles, J. Biol. Chem. 251, 277–284.
McCabe, E.R.B. (2001) Disorders of Glycerol Metabolism, in The Metabolic and Molecular Bases of Inherited Disease (Scriver, C., Beaudet, A.L., Sly, W.S., Valle, D., Childs, B., Kinzler, K.W., and Vogelstein, B., eds.), 8th edn., pp. 2217–2237, McGraw-Hill, New York.
Walker, A.P., Muscatelli, F., Stafford, A.N., Chelly, J., Dahl, N., Blomquist, H.K., Delanghe, J., Willems, P.J., Steinmann, B., and Monaco, A.P. (1996) Mutations and Phenotype in Isolated Glycerol Kinase Deficiency, Am. J. Hum. Genet. 58, 1205–1211.
Dipple, K.M., Zhang, Y.-H., Huang, B.-L., McCabe, L.L., Dallongeville, J., Inokuchi, T., Kimura, M., Marx, H.J., Roederer, G.O., Shih, V., Yamaguchi, S., Yoshida, I., and McCabe, E.R.B., (2001) Glycerol Kinase Deficiency: Evidence for Complexity in a Single Gene Disorder, Hum. Genet. 109, 55–62.
Hellerud, C., Burlina, A., Gabelli, C., Ellis, J.R., Nyholm, P.G., and Lindstedt, S. (2003) Glycerol Metabolism and the Determination of Triglycerides—Clinical, Biochemical and Molecular Findings in Six Subjects, Clin. Chem. Lab. Med. 41, 46–55.
Hellerud, C., Adamowicz, M., Jurkiewicz, D., Taybert, J., Kubalska, J., Ciara, E., Popowska, E., Ellis, J.R., Lindstedt, S., and Pronicak, E. (2003) Clinical Heterogeneity and Molecular Findings in Five Polish Patients with Glycerol Kinase Deficiency: Investigation of Two Splice Site Mutations with Computerized Splice Junction Analysis and Xp21 Gene-Specific mRNA Analysis, Mol. Genet. Metab. 79, 149–159.
Mahbubul Huq, A.H., Lovell, R.S., Ou, C.-N., Beaudet, A.L., and Craigen, W.J. (1997) X-Linked Glycerol Kinase Deficiency in the Mouse Leads to Growth Retardation, Altered Fat Metabolism, Autonomous Glucocorticoid Secretion and Neonatal Death, Hum. Mol. Genet. 6, 1803–1809.
Bartley, J.A., and Ward, R. (1985) Glycerol Kinase Deficiency Inhibits Glycerol Utilization in Phosphoglyceride and Triacylglycerol Biosynthesis, Pediatr. Res. 19, 313–314.
Hara, A., and Radin, N.S. (1978) Lipid Extraction of Tissues with a Low-Toxicity Solvent, Anal. Biochem. 90, 420–426.
Saunders, R.D., and Horrocks, L.A. (1984) Simultaneous Extraction and Preparation for HPLC of Prostaglandins and Phospholipids, Anal. Biochem. 143, 71–75.
Dugan, L.L., Demediuk, P., Pendley, C.E., II, and Horrocks, L.A. (1986) Separation of Phospholipids by High Pressure Liquid Chromatography: All Major Classes Including Ethanolamine and Choline Plasmalogens, and Most Minor Classes, Including Lysophosphatidylethanolamine, J. Chromatogr. 378, 317–327.
Murphy, E.J., Stephens, R., Jurkowitz-Alexander, M., and Horrocks, L.A. (1993) Acidic Hydrolysis of Plasmalogens Followed by High-Performance Liquid Chromatography, Lipids 28, 565–568.
Rouser, G., Siakotos, A., and Fleischer, S. (1969) Quantitative Analysis of Phospholipids by Thin Layer Chromatography and Phosphorus Analysis of Spots, Lipids 1, 85–86.
Murphy, E.J., Prows, D.R., Jefferson, J.R., and Schroeder, F. (1996) Liver Fatty Acid Binding Protein Expression in Transfected Fibroblasts Stimulates Fatty Acid Uptake and Metabolism, Biochim. Biophys. Acta 1301, 191–196.
Murphy, E.J., Rosenberger, T.A., and Horrocks, L.A. (1996) Separation of Neutral Lipids by High Performance Liquid Chromatography: Quantification by Ultraviolet, Light Scattering and Fluorescent Detectors, J. Chromatogr. B 685, 9–14.
Akesson, B., Elovsson, J., and Arvidsson, G. (1970) Initial Incorporation into Rat Liver Glycerolipids of Intraportally Injected [3H]Glycerol, Biochim. Biophys. Acta 210, 15–27.
Murphy, E.J., and Schroeder, F. (1997) Sterol Carrier Protein-2 Mediated Cholesterol Esterification in Transfected L-Cell Fibroblasts, Biochim. Biophys. Acta 1345, 283–292.
Brockerhoff, H. (1975) Determination of the Positional Distribution of Fatty Acids in Glycerolipids. Methods Enzymol. 35, 315–325.
Bradford, M. (1976) A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding, Anal. Biochem. 72, 248–254.
Brown, L.J., Kosa, R.A., Marshall, L., Kozak, L.P., and Mac-Donald, M.J. (2002) Lethal Hypoglycemic Ketosis and Glyceroluria in Mice Lacking Both the Mitochondrial and the Cytosolic Glycerol Phosphate Dehydrogenases, J. Biol. Chem. 277, 32899–32904.
Custo, G., Corazzi, L., Mastrofini, P., and Arienti, G. (1987) Glycerol Incorporation into Brain Lipids in Rat Pups Born to Ethanol-Intoxicated Dams, Neurochem. Res. 12, 469–473.
Tardi, P.G., Man, R.Y., and Choy, P.C. (1992) The Effect of Methyl-Lidocaine on the Biosynthesis of Phospholipids de novo in the Isolated Hamster Heart, Biochem. J. 285, 161–166.
Athenstaedt, K., and Daum, G. (1999) Phosphatidic Acid, a Key Intermediate in Lipid Metabolism, Eur. J. Biochem. 266, 1–16.
Watford, M. (2000) Functional Glycerol Kinase Activity and the Possibility of a Major Role for Glyceroneogenesis in Mammalian Skeletal Muscle, Nutr. Rev. 58, 145–148.
Crabtree, B., and Newsholme, E.A. (1972) The Activities of Phosphorylase, Hexokinase, Phosphofructokinase, Lactate Dehydrogenase and the Glycerol 3-Phosphate Dehydrogenases in Muscles from Vertebrates and Invertebrates, Biochem. J. 126, 49–58.
Duelli, R., Maurer, M.H., Staudt, R., Heiland, S., Duembgen, L., and Kuschinsky, W. (2000) Increased Cerebral Glucose Utilization and Decreased Glucose Transporter Glut1 During Chronic Hyperglycemia in Rat Brain, Brain Res. 858, 338–347.
Sokoloff, L., Reivich, M., Kennedy, C., Des Roisiers, M.H., Patlak, C.S., Pettigrew, K.D., Sakurada, O., and Shinohara, M. (1977) The [14C]Deoxyglucose Method for the Measurement of Local Cerebral Glucose Utilization: Theory, Procedure, and Normal Values in the Conscious and Anesthetized Albino Rat, J. Neurochem. 28, 897–916.
Garcia-Salguero, L., and Lupianez, J.A. (1989) Metabolic Adaptation of the Renal Carbohydrate Metabolism. II. Effects of a High Carbohydrate Diet on the Gluconeogenic and Glycolytic Fluxes in the Proximal and Distal Renal Tubules, Mol. Cell. Biochem. 85, 91–100.
Lee, D.P., Deonarine, A.S., Kienetz, M., Zhu, Q., Skrzypczak, M., Chan, M., and Choy, P.C. (2001) A Novel Pathway for Lipid Biosynthesis: The Direct Acylation of Glycerol, J. Lipid Res. 42, 1979–1986.
Ma, T., Yang, B., and Verkman, A.S. (1997) Cloning of a Novel Water and Urea-Permeable Aquaporin from Mouse Expressed Strongly in Colon, Placenta, Liver, and Heart, Biochem. Biophys. Res. Commun. 240, 324–328.
DeGrella, R.F., and Light, R.J. (1980) Uptake and Metabolism of Fatty Acids by Dispersed Adult Rat Heart Myocytes. I. Kinetics of Homologous Fatty Acids, J. Biol. Chem. 255, 9731–9738.
Klein, M.S., Goldstein, R.A., Welch, M.J., and Sobel, B.E. (1979) External Assessment of Myocardial Metabolism with [11C]Palmitate in Rabbit Hearts, Am. J. Physiol. 237, H51-H57.
Tamboli, A., O'Looney, P., Vander Maten, M., and Vahouny, G.V. (1983) Comparative Metabolism of Free and Esterified Fatty Acids by the Perfused Rat Heart and Rat Cardiac Myocytes, Biochim. Biophys. Acta 750, 404–410.
McMaster, C.R., and Bell, R.M. (1997) CDP-Choline: 1,2-Diacylglycerol Cholinephosphotransferase, Biochim. Biophys. Acta 1348, 100–110.
McMaster, C.R., and Bell, R.M. (1997) CDP-Ethanolamine:1,2-Diacylglycerol Ethanolaminephosphotransferase, Biochim. Biophys. Acta 1348, 117–123.
Araki, W., and Wurtman, R.J. (1998) How Is Membrane Phospholipid Biosynthesis Controlled in Neural Tissues? J. Neurosci. Res. 51, 667–674.
Heacock, A.M., and Agranoff, B.W. (1997) CDP-Diacylglycerol Synthase from Mammalian Tissues, Biochim. Biophys. Acta 1348, 166–172.
Schroeder, F., Frolov, A.A., Murphy, E.J., Atshaves, B.P., Pu, L., Wood, W.G., Foxworth, W.B., and Kier, A.B. (1996) Recent Advances in Membrane Cholesterol Domain Dynamics and Intracellular Cholesterol Trafficking, Proc. Soc. Exp. Biol. Med. 213, 150–177.
Wood, W.G., Schroeder, F., Avdulov, N.A., Chochina, S.V., and Igbauboa, U. (1999) Recent Advances in Brain Cholesterol Dynamics: Transport, Domains, and Alzheimer's Disease, Lipids 34, 225–234.
Petrovich, D.R., Finkelstein, S., Waring, A.J., and Farber, J.L. (1984) Liver Ischemia Increases the Molecular Order of Microsomal Membranes by Increasing the Cholesterol-to-Phospholipid Ratio, J. Biol. Chem. 259, 13217–13223.
Murphy, E.J., Chapiro, M.B., Rapoport, S.I., and Shetty, H.U. (2000) Phospholipid Composition and Levels Are Altered in Down Syndrome Brain, Brain Res. 867, 9–18.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Golovko, M.Y., Hovda, J.T., Cai, ZJ. et al. Tissue-dependent alterations in lipid mass in mice lacking glycerol kinase. Lipids 40, 287–293 (2005). https://doi.org/10.1007/s11745-005-1384-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-005-1384-2