Abstract
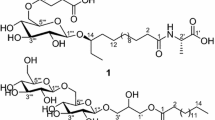
Two antibacterial and xanthine oxidase inhibitory cerebrosides, one of which is chemically new, were characterized from the chloroform-methanol (1∶1) extract of Fusarium sp. IFB-121, an endophytic fungus in Quercus variabilis. By means of chemical and spectral methods [IR, electrospray ionization MS (ESI-MS), tandem ESI-MS, 1H and 13C NMR, distortionless enhancement by polarization transfer, COSY, heteronuclear multiple-quantum coherence, heteronuclear multiple-bond correlation, and 2-D nuclear Overhauser effect correlation spectroscopy], the structure of the new metabolite named fusaruside was established as (2S,2′R,3R,3′E,4E,8E,10E)-1-O-β-d-glucopyranosyl-2-N-(2′-hydroxy-3′-octadecenoyl)-3-hydroxy-9-methyl-4,8,10-sphingatrienine, and the structure of the other was identified as (2S,2′R,3R,3′E,4E,8E)-1-O-β-d-glucopyranosyl-2-N-(2′-hydroxy-3′-octadecenoyl)-3-hydroxy-9-methyl-4,8-sphingadienine. Both new and known cerebrosides, although inactive to Trichophyton rubrum and Candida albicans, showed strong antibacterial activities against Bacillus subtilis, Escherichia coli, and Pseudomonas fluorescens, with their minimum inhibitory concentrations being 3.9, 3.9, and 1.9 μg/mL, and 7.8, 3.9, and 7.8 μg/mL, respectively. Furthermore, both metabolites were inhibitory to xanthine oxidase, with the IC50 value of fusaruside being 43.8±3.6 μM and the known cerebroside being 55.5±1.8 μM.
Similar content being viewed by others
Abbreviations
- ESI-MS:
-
electrospray ionization MS
- ESI-MS/MS:
-
tandem electrospray ionization MS
- HMBC:
-
heteronuclear multiple-bond correlation
- HMQC:
-
heteronuclear multiple-quantum coherence
- HR-ESI-MS:
-
high-resolution electrospray ionization MS
- MIC:
-
minimum inhibitory concentration
- NOESY:
-
2-D nuclear Overhauser effect correlation spectroscopy
- PDA:
-
potato-dextrose-agar
- XO:
-
xanthine oxidase
References
Tan, R.X., and Chen, J.H. (2003) The Cerebrosides, Nat. Prod. Rep. 20, 509–534.
Liu, J.Y., Liu, C.H., Zou, W.X. Tian, X., and Tan, R.-X. (2002) Leptosphaerone, a Metabolite with a Novel Skeleton from Leptosphaeria sp. IV403, an Endophytic Fungus in Artemisia annua, Helv. Chim. Acta 85, 2664–2667.
Tan, R.X., and Zou, W.X. (2001) Endophytes: A Rich Source of Functional Metabolites, Nat. Prod. Rep. 18, 448–459.
Zou, W.X., Lu, H., Meng, J.C., Chen, G.X., Zhang, T.Y., and Tan, R.X. (2000) New and Bioactive Metabolites of Colletotrichum gloeosporioides, an Endophytic Fungus in Artemisia mongolica, J. Nat. Prod. 63, 1529–1530.
Lu, H., Zou, W.X., Meng, J.C., Hu, J., and Tan, R.X. (2000) New Bioactive Metabolites Produced by Colletotrichum sp., an Endophytic Fungi in Artemisia annua, Plant Sci. 151, 67–73.
Dickson, R.-C., and Lester, R.-L. (1999) Yeast Sphingolipids, Biochim. Biophys. Acta 1426, 347–357.
Batrakov, S.G., Konova, I.V., Sheichenko, V.I., Esipov, S.E., Galanina, L.A., and Istratova, L.N. (2002) Unusual Fatty Acid Composition of Cerebrosides from the Filamentous Soil Fungus Mortierella alpina, Chem. Phys. Lipids 117, 45–51.
Chen, J.H., Cui, G.Y., Liu, J.Y., and Tan, R.X. (2003) Pinelloside, an Antimicrobial Cerebroside from Pinellia ternate, Phytochemistry 64, 903–906.
Qi, J., Ojika, M., and Sakagami Y. (2000) Termitomycesphins A-D, Novel Neuritogenic Cerebrosides from the Edible Chinese Mushroom Termitomyces albuminosus, Tetrahedron 56, 5835–5841.
Gao, J.M., Hu, L., Dong, Z.J., and Liu, J.K. (2001) New Glycosphingolipid Containing an Unusual Sphingoid Base from the Basidiomycete Polyporus ellisii, Lipids 36, 521–527.
Kong, L.D., Abliz, Z., Zou, C.X., Li, L.J., Cheng, C.H.K., and Tan, R.X. (2001) Glycosides and Xanthine Oxidase Inhibitors from Conyzaa bonariensis, Phytochemistry 58 645–651.
Van Hoorn, D.E.C., Nijveldt, R.J., Van Leeuwen, P.A.M., Hofman, Z., M'Rabet, L., De Bont, D.B.A., and Van Norren, K. (2002) Accurrate Prediction of Xanthine Oxidase Inhibition Based on the Structure of Flavonoids, Eur. J. Pharmacol. 451, 111–118.
Toledo, M.S., Levery, S.B., Straus, A.H., Suzuki, E., Momany, M., Glushka, J., Moulton, J.M., and Takahashi H.K. (1999) Characterization of Sphingolipids from Mycopathogens: Factors Correlating with Expression of 2-Hydroxy Fatty Acyl Δ3-Unsaturation in Cerebrosides of Paracoccidioides brasiliensis and Aspergillus fumigatus, Biochemistry 38, 7294–7306.
Duarte, R.S., Polycarpo, C.R., Wait, R., Hartmann, R., and Bergter, E.B. (1998) Structural Characterization of Neutral Glycosphingolipids from Fusarium Species, Biochim. Biophys. Acta 1390, 186–196.
Keusgen, M., Yu, C.-M. Curtis, J.M., Brewer, D., and Ayer, S.W. (1996) A Cerebroside from the Marine Fungus Microsphaeropsis olivacea (Bonord.) Höhn, Biochem. Syst. Ecol. 24, 465–468.
Umemura, K., Ogawa, N., Yamauchi, T., Iwata, M., Shimura, M., and Koga, J. (2000) Cerebroside Elicitors Found in Diverse Phytopathogens Activate Defense Responses in Rice Plants, Plant Cell Physiol. 41, 676–683.
Huang, Y., Dong, Z.J., and Liu, J.K. (2001) The Chemical Constituents from Basidiocarps of Sarcodon aspratum, Acta Bot. Yunnanica 23, 125–128.
Zhan, Z.J., Sun, H.D., Wu, H.M., and Yue, J.M. (2003) Chemical Components from the Fungus Engleromyces goetzei, Acta Bot. Sin. 45, 248–252.
Kong, L.D., Cai Y., Huang, W.W., Cheng, C.H.K., and Tan, R.X. (2000) Inhibition of Xanthine Oxidase by Some Chinese Medicinal Plants Used to Treat Gout, J. Ethnopharmacol. 73, 199–207.
Barrett, A.G.M., Beall, J.C., Braddock, D.C., Flack, K., Gibson, V.C., and Salter, M.M. (2000) Asymmetric Allylboration and Ring Closing Alkene Metathesis: A Novel Strategy for the Synthesis of Glycosphingolipids, J. Org. Chem. 65, 6508–6514.
Tan, J.W., Dong, Z.J., and Liu, J.K. (2003) New Cerebrosides from the Basidiomycete Cortinarius tenuipes, Lipids 38, 81–84.
Boas, M.H.S.V., Egge, H., Pohlentz, G., Hartmann, R., and Bergter, E.B. (1994) Structural Determination of N-2′-Hydroxyoctadecenoyl-1-O-β-d-glucopyranosyl-9-methyl-4,8-sphingadienine from Species of Aspergillus, Chem. Phys. Lipids 70, 11–19.
Kawai, G. (1989) Molecular Species of Cerebrosides in Fruiting Bodies of Lentimus edodes and Their Biological Activity, Biochim. Biophys. Acta 1001, 185–190.
Sitrin, R.D., Chan, G., Dinger-dissen, J., DeBrosse, C., Mehta, R., Roberts, G., Rottschaefer, S., Staiger, D., Valenta, J., Snader, K.M., et al. (1988) Isolation and Structure Determination of Pachybasium Cerebrosides Which Potentiate the Antifungal Activity of Aculeacin, J. Antibiot. 41, 469–480.
Nishida, F., Mori, Y., Rokkaku, N., Isobe, S., Furuse, T., Suzuki, M., Meevootisom, V., Flegel, T.W., Thebtaranonth, Y., and Intararuangsorn, S. (1990) Structure Elucidation of Glycosidic Antibiotics Glykenins from Basidiomycetes sp. II. Absolute Structures of Unusual Polyhydroxylated C26-Fatty Acids, Aglycones of Glykenins, Chem. Pharm. Bull. 38, 2381–2389.
Nishida, F., Mori Y., Sonobe, C., and Suzuki, M. (1991) Structure Elucidation of Glycosidic Glykenins from Basidomycetes sp. III. J. Antibiot. 44, 541–545.
da Silva, A.F.C., Rodrigues, M.L., Farias, S.E., Almeida, I.C., Pinto M.R., and Barreto-Bergter, E. (2004) Glucosylceramides in Colletotrichum gloeosporioides Are Involved in the Differentiation of Conidia into Mycelial Cells, FEBS Lett. 561, 137–143.
Weiss, B., Stiller, R.L., and Jack, P.C. (1973) Sphingolipids of the Fungi Phycomycetes blakesleeanus and Fusarium lini, Lipids 8, 25–30.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Shu, R.G., Wang, F.W., Yang, Y.M. et al. Antibacterial and xanthine oxidase inhibitory cerebrosides from Fusarium sp. IFB-121, and endophytic fungus in Quercus variabilis . Lipids 39, 667–673 (2004). https://doi.org/10.1007/s11745-004-1280-9
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-004-1280-9