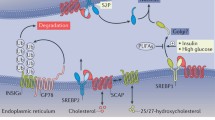
Abstract
The origins of cholesterol research can be traced to prerevolutionary France. The discovery of cholesterol as a single substance, present in human gallstones, owes much to the scientists of l'Académie Française, including Lavoisier, who contributed so much to the emergence of chemistry as a modern scientific discipline. Since that time, cholesterol probably has been the most intensively scrutinized natural product of all time, and it has been the subject of Nobel Prizes for several who have studied its structure, biosynthesis, and regulation. The pace of research into cholesterol shows no sign of diminishing, and recent discoveries have led to the recognition that the regulation of cholesterol metabolism is intimately linked with that of other metabolic pathways. Details of these interactions are only just emerging, but it is becoming apparent that under some circumstances it is difficult to reconcile, in a conventional manner, changes in regulatory gene expression with corresponding changes in pathway carbon flux. The present review includes some of our studies on the roles of the transcription factors sterol regulatory element-binding protein, liver X-receptor α, and peroxisome proliferator activated receptor α in the coordination of cholesterol and fatty acid synthesis and describes how some of the results obtained can best be interpreted from a Metabolic Control Analysis perspective of the regulation of pathway carbon fluxes.
Similar content being viewed by others
Abbreviations
- CYP7A1:
-
cholesterol 7α-hydroxylase
- ER:
-
endoplasmic reticulum
- FAS:
-
fatty acid synthase
- LXRα:
-
liver X-receptor α
- MCA:
-
Metabolic Control Analysis
- PPARα:
-
peroxisome proliferator-activated receptor α
- SCAP:
-
SREBP cleavage-activating protein
- SREBP:
-
sterol regulatory element-binding protein
References
Cornforth, J.W. (1976) Asymmetry and Enzyme Action. in Les Prix Nobel en 1975, pp. 119–132, Nobel Foundation, Stockholm.
Popják, G. (1977) “As I Remember It.” Research on Biosynthesis of Fatty Acids, Triglycerides, Squalene and Cholesterol, J. Am. Oil Chem. Soc. 54, 647A-659A.
Schroepfer, G.F., Jr. (2000) Oxysterols: Modulators of Cholesterol Metabolism and Other Processes, Physiol. Rev. 80, 361–554.
Forman, B.M., Ruan, B., Chen, J., Schroepfer, G.J., Jr., and Evans, R.M. (1997) The Orphan Nuclear Receptor LXRα Is Positively and Negatively Regulated by Distinct Products of Mevalonate Metabolism, Proc. Natl. Acad. Sci. USA 94, 10588–10593.
Chevreul, M.E. (1815) Ann. Chim. Phys. 95, 5.
Smeaton, W.A. (1962) Fourcroy: Chemist and Revolutionary 1755–1809, pp. 53–59, Heffer, London.
Fourcroy, A. (1790) Memoire sur les differens états de cadavres trouves dans les fouilles du cimetiere des Innocens en 1786 et 1787, Ann. Chim. 5, 154–185.
Fourcroy, A. (1789) Examen chimique de la substance fueilletee et cristalline continue dans les calculs biliares; et de la nature des concretions cystiques cristallisees, Ann. Chim. 3, 242–252.
Chevreul, M.E. (1816) Examen des graisses d'homme, de mouton, de boeuf, de jaguar et d'oie, Ann. Chim. Phys. 2, 339–372.
Windaus, A. (1910) Über den Gehalt normaler und athermatöser Aorten an Cholesterin und Cholesterinester, Z. Physiol. Chem. 67, 174–184.
Krebs, H.A. (1981) Reminiscences and Reflections, Oxford University Press, Oxford, United Kingdom.
Bernal, J.D. (1932) Carbon Skeleton of the Sterols, Chem. Ind. 51, 466.
Perutz, M. (2001) The Great Sage, Chem. Br., 37, 56–57.
Wieland, H., and Dane, E. (1932) Untersuchungen über die Konstitution der Gallensäuren. 39. Mitteilung. Zur Kenntnis der 12-oxy-cholansäure, Zt. Physiol. Chem. 210, 268–281.
Rosenheim, O., and King, H. (1932) The Ring System of Sterols and Bile Acids, Nature 130, 315.
Florkin, M., and Stotz, E.H. (1979) History of Biochemistry, Comp. Biochem. 33A, 1–26.
Cornforth, J.W. (2002) Sterol Biosynthesis: The Early Days, Biochem. Biophys. Res. Commun. 292, 1129–1138.
Gould, R.G., Taylor, C.B., Hagerman, J.S., Warner, I., and Campbell, D.J. (1953) Cholesterol Metabolism. I. Effect of Dietary Cholesterol on the Synthesis of Cholesterol in Dog Tissues in vitro, J. Biol. Chem. 201, 519–528.
Horton, J.D., Goldstein, J.L., and Brown, M.S. (2002) SREBPs: Activators of the Complete Program of Cholesterol and Fatty Acid Synthesis in the Liver, J. Clin. Invest. 109, 1125–1131.
Ingham, P.W. (2001) Hedgehog Signaling: A Tale of Two Lipids, Science 294, 1879–1881.
Kandutsch, A.A., and Chen, H.W. (1973) Inhibition of Sterol Synthesis in Cultured Mouse Cells by 7α-Hydroxycholesterol, 7β-Hydroxycholesterol and 7-Ketocholesterol, J. Biol. Chem. 248, 8408–8417.
Fell, D., ed. (1997) Understanding the Control of Metabolism, Frontiers in Metabolism Series 2 (Snell, K., series ed.), 300 pp., Portland Press, London.
Janowski, B.A., Willy, P.J., Devi, T.R., Falck, J.R., and Mangelsdorf, D.J. (1996) An Oxysterol Signalling Pathway Mediated by the Nuclear Receptor LXRα, Nature 383, 728–731.
Lehmann, J.M., Kliewer, S.A., Moore, L.B., Smith-Oliver, T.A., Oliver, B.B., Su, J.L., Sundseth, S.S., Winegar, D.A., Blanchard, D.E., Spencer, T.A., and Willson, T.M. (1997) Activation of the Nuclear Receptor LXR by Oxysterols Defines a New Hormone Response Pathway, J. Biol. Chem. 272, 3137–3140.
Repa, J.J., Turley, S.D., Lobaccaro, J.A., Medina, J., Li, L., Lustig, K., Shan, B., Heyman, R.A., Dietschy, J.M., and Mangelsdorf, D.J. (2000) Regulation of Absorption and ABC1-Mediated Efflux of Cholesterol by RXR Heterodimers, Science 289, 1524–1529.
Schultz, J.R., Tu, H., Luk, A., Repa, J.J., Medina, J.C., Li, L., Schwendner, S., Wang, S., Thoolen, M., Mangelsdorf, D.J., Lustig, K.D., and Shan, B. (2000) Role of LXRs in Control of Lipogenesis, Genes Dev. 14, 2831–2838.
Saucier, S.E., Kandutsch, A.A., Gayen, A.K., Swahn, D.K., and Spencer, T.A. (1989) Oxysterol Regulators of 3-Hydroxy-3-methylglutaryl-CoA Reductase in Liver. Effect of Dietary Cholesterol, J. Biol. Chem. 264, 6863–6869.
Repa, J.J., Liang, G., Ou, J., Bashmakov, Y., Lobaccaro, J.M., Shimomura, I., Shan, B., Brown, M.S., Goldstein, J.L., and Mangelsdorf, D.J. (2000) Regulation of Mouse Sterol Regulatory Element-Binding Protein-1c Gene (SREBP-1c) by Oxysterol Receptors, LXRα and LXRβ, Genes Dev. 14, 2819–2830.
Matsumoto, M., Ogawa, W., Teshigawara, K., Inoue, H., Miyake, K., Sakaue, H., and Kasuga, M., (2002) Role of the Insulin Receptor Substrate 1 and Phosphatidylinositol 3-Kinase Signaling Pathway in Insulin-Induced Expression of Sterol Regulatory Element Binding Protein 1c and Glucokinase Genes in Rat Hepatocytes, Diabetes 51, 1672–1680.
Shimomura, I., Matsuda, M., Hammer, R.E., Bashmakov, Y., Brown, M.S., and Goldstein, J.L. (2000) Decreased IRS-2 and Increased SREBP-1c Lead to Mixed Insulin Resistance and Sensitivity in Livers of Lipodystrophic and ob/ob Mice, Mol. Cell. 6, 77–86.
Fleischmann, M., and Iynedjian, P.B. (2000) Regulation of Sterol Regulatory-Element Binding Protein 1 Gene Expression in Liver: Role of Insulin and Protein Kinase B/cAkt, Biochem. J. 349, 13–17.
Shimomura, I., Bashmakov, Y., Ikemoto, S., Horton, J.D., Brown, M.S., and Goldstein, J.L. (1999) Insulin Selectively Increases SREBP-1c mRNA in the Livers of Rats with Streptozotocin-Induced Diabetes, Proc. Natl. Acad. Sci. USA 96, 13656–13661.
Foretz, M., Pacot, C., Dugail, I., Lemarchand, P., Guichard, C., Le-Liepvre, X., Berthelier-Lubrano, C., Spiegelman, B., Kim, J.B., Ferré, P., and Foufelle, F. (1999) ADD1/SREBP-1c Is Required in the Activation of Hepatic Lipogenic Gene Expression by Glucose, Mol. Cell Biol. 19, 3760–3768.
Gibbons, G.F., Patel, D.D., Wiggins, D., and Knight, B.L. (2002) The Functional Efficiency of Lipogenic and Cholesterogenic Gene Expression in Normal Mice and in Mice Lacking the Peroxisomal Proliferator-Activated Receptor α (PPAR-α), Adv. Enzyme Regul. 42, 227–247.
Jungas, R.L. (1968) Fatty Acid Synthesis in Adipose Tissue Incubated in Tritiated Water, Biochemistry 7, 3708–3717.
Gibbons, G.F., Björnsson, O.G., and Pullinger, C.R. (1984) Evidence That Changes in Hepatic 3-Hydroxy-3-methylglutaryl Coenzyme A Reductase Activity Are Required Partly to Maintain a Constant Rate of Sterol Synthesis, J. Biol. Chem. 259, 14399–14405.
Patel, D.D., Knight, B.L., Wiggins, D., Humphreys, S.M., and Gibbons, G.F. (2001) Disturbances in the Normal Regulation of SREBP-Sensitive Genes in PPARα-Deficient Mice, J. Lipid Res. 42, 328–333.
Sugden, M.C., Bulmer, K., Gibbons, G.F., Knight, B.L., and Holness, M.J. (2002) Peroxisome-Proliferator-Activated Receptor-α (PPAR-α) Deficiency Leads to Dysregulation of Hepatic Lipid and Carbohydrate Metabolism by Fatty Acids and Insulin, Biochem. J. 364, 361–368.
de Vasconcelos, P.R.L., Kettlewell, M.G.W., Gibbons, G.F., and Williamson, D.H. (1989) Increased Rates of Hepatic Cholesterogenesis and Lipogenesis in Septic Rats in vivo. Evidence for the Possible Involvement of Insulin, Clin. Sci. 76, 205–211.
Siperstein, M.D., and Guest, M.J. (1960) Studies on the Site of the Feedback Control of Cholesterol Synthesis, J. Clin. Invest. 39, 642–652.
Shimano, H. (2001) Sterol Regulatory Element-Binding Proteins (SREBPs): Transcriptional Regulators of Lipid Synthetic Genes, Prog. Lipid Res. 40, 439–452.
Shimomura, I., Bashmakov, Y., Shimano, H., Horton, J.D., Goldstein, J.L., and Brown, M.S. (1997) Cholesterol Feeding Reduces Nuclear Forms of Sterol Regulatory Element Binding Proteins in Hamster Liver, Proc. Natl. Acad. Sci. USA 94, 12354–12359.
Wang, X., Sato, R., Brown, M.S., Hua, X., and Goldstein, J.L. (1994) SREBP-1, a Membrane-Bound Transcription Factor Released by Sterol-Regulated Proteolysis, Cell 77, 53–62.
Peet, D.J., Turley, S.D., Ma, W., Janowski, B.A., Lobaccaro, J.M., Hammer, R.E., and Mangelsdorf, D.J. (1998) Cholesterol and Bile Acid Metabolism Are Impaired in Mice Lacking the Nuclear Oxysterol Receptor LXRα, Cell 93, 693–704.
Fungwe, T.V., Cagen, L.M., Wilcox, H.G., and Heimberg, M. (1994) Effects of Dietary Cholesterol on Hepatic Metabolism of Free Fatty Acid and Secretion of VLDL in the Hamster, Biochem. Biophys. Res. Commun. 200, 1505–1511.
Fungwe, T.V., Fox, J.E., Cagen, L.M., Wilcox, H.-G., and Heimberg, M. (1994) Stimulation of Fatty Acid Biosynthesis by Dietary Cholesterol and of Cholesterol Synthesis by Dietary Fatty Acid, J. Lipid Res. 35, 311–318.
Reddy, J.K., and Hashimoto, T. (2001) Peroxosomal β-Oxidation and Peroxisome Proliferator-Activated Receptor α: An Adaptive Metabolic System, Annu. Rev. Nutr. 21, 193–230.
Fruchart, J.C., Duriez, P., and Staels, B. (1999) Peroxisome Proliferator-Activated Receptor-α Activators Regulate Genes Governing Lipoprotein Metabolism, Vascular Inflammation and Atherosclerosis, Curr. Opin. Lipidol. 10, 245–257.
Brown, M.S., Goldstein, J.L., and Dietschy, J.M. (1979) Active and Inactive Forms of 3-Hydroxy-3-methylglutaryl Coenzyme A Reductase in the Liver of the Rat. Comparison with the Rate of Cholesterol Synthesis in Different Physiological States, J. Biol. Chem. 254, 5144–5149.
Edwards, P.A., Fogelman, A.M., and Tanaka, R.D. (1983) Physiological Control of HMG-CoA Reductase, in 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase (Sabine, J.R., ed.), pp. 93–105, CRC Press, Boca Raton.
Shulman, R.G., and Rothman, D.L. (2001) 13C NMR of Intermediary Metabolism: Implications for Systemic Physiology, Annu. Rev. Physiol. 63, 15–48.
Wildermuth, M.C. (2000) Metabolic Control Analysis: Biological Applications and Insights, Genome Biol. 1, 1031.
Bowden, A.C. (1999) Metabolic Control Analysis in Biotechnology and Medicine, Nat. Biotechnol. 17, 641–643.
Pullinger, C.R., and Gibbons, G.F. (1983) The Role of Substrate Supply in the Regulation of Cholesterol Biosynthesis in Rat Hepatocytes, Biochem. J. 210, 625–632.
Gibbons, G.F., Pullinger, C.R., Munday, M.R., and Williamson, D.H. (1983) Regulation of Cholesterol Synthesis in the Liver and Mammary Gland of the Lactating Rat, Biochem. J. 212, 843–848.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Gibbons, G.F. From gallstones to genes: Two hundred years of sterol research. A tribute to George J. Schroepfer Jr.. Lipids 37, 1153–1162 (2002). https://doi.org/10.1007/s11745-002-1015-y
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-002-1015-y