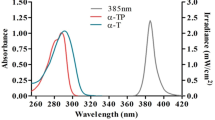
Abstract
α-Tocopherol (α-TH) undergoes ultraviolet (UV)-induced photooxidation on the surface of mouse skin to produce a dihydroxydimer, a spirodimer, and trimers as the major products. To study the photochemistry involved, we UV-irradiated α-TH in a thin film on a glass petri dish. Photooxidation yielded a mixture of dihydroxydimer, spirodimer, and trimers. In the time-course studies, the dihydroxydimer accumulated and then was further oxidized, whereas the spirodimer and trimers accumulated more gradually. Reaction of two tocopheroxyl radicals forms the dihydroxydimer, whereas the spirodimer may be formed either by photooxidation of α-TH to an orthoquinone methide (o-QM) followed by a Diels-Alder reaction or by photooxidation of α-TH to the dihydroxydimer, followed by two-electron oxidation. Irradiation of a mixture of d 10-labeled and unlabeled (d 0) dihydroxydimer produced a mixture of labeled and unlabeled spirodimers as detected by positive atmospheric pressure chemical ionization-mass spectrometry. The absence of mixed label spirodimers among products indicated that direct oxidation of the dihydroxydimer is a facile route to the spirodimer and is probably the major spirodimer-forming reaction in α-TH photooxidations. Trimer formation from the dihydroxydimer and the spirodimer was observed, however, and requires an o-QM intermediate. Photooxidation of d 10-labeled and unlabeled (d 0) dihydroxydimers yielded mixed isotopomers of the trimer products, thus demonstrating that the dihydroxydimer and spirodimers underwent conversion to o-QM intermediates. Photochemical conversion of α-TH to UV-absorbing dimer and trimer products may contribute to photoprotection by topically applied α-TH.
Similar content being viewed by others
Abbreviations
- APCI-MS:
-
atmospheric pressure chemical ionization mass spectrometry
- ESI-MS:
-
electrospray ionization-mass spectrometry
- MS-MS:
-
tandem mass spectrometry
- o-QM:
-
orthoquinone methide
- RP-HPLC:
-
reversed-phase high-performance liquid chromatography
- α-TAc:
-
α-tocopherol acetate
- α-TH:
-
α-tocopherol
- UV-vis:
-
ultraviolet-visible
References
Kramer, K.A., and Liebler, D.C. (1997) UVB-Induced Photooxidation of Vitamin E, Chem. Res. Toxicol. 10, 219–224.
McVean, M., and Liebler, D.C. (1997) Inhibition of UVB-Induced Photodamage in Mouse Epidermis by Topically Applied α-Tocopherol, Carcinogenesis, 18, 1617–1622.
McVean, M., and Liebler, D.C. (1999) Prevention of DNA Photodamage by Vitamin E Compounds and Sunscreens: Roles of Ultraviolet Absorbance and Cellular Uptake, Mol. Carcinog. 24, 169–176.
Kramer-Stickland, K.A., Krol, E.S., and Liebler, D.C. (1999) UV-B-Induced Photooxidation of Vitamin E in Mouse Skin, Chem. Res. Toxicol. 12, 187–191.
Skinner, W.A., and Alaupovic, P. (1963) Oxidation Products of Vitamin E and Its Model, 6-Hydroxy-2,2,5,7,8-pentamethyl-chroman. V. Studies of the Products of Alkaline Ferricyanide Oxidation, J. Org. Chem. 28, 2854–2858.
Skinner, W.A., and Parkhurst, R.M. (1963) Oxidation Products of Vitamin E and Its Model 6-Hydroxy-2,2,5,7,8-Pentamethylchroman. VIII. Oxidation with Benzoyl Peroxide, J. Org. Chem. 31, 1248–1251.
Nilsson, J.L.G., Sievertssom, H., and Selander, H. (1968) Tocopherols VIII. New Dimers and Spiroketal Trimers as Oxidation Products of Tocol Derivatives, Tetrahedron Lett. 48, 5023–5025.
Suarna, C., Craig, D.C., Cross, K.J., and Southwell-Keely, P.T. (1988) Oxidations of Vitamin E (α-tocopherol) and Its Model Compound 2,3,5,7,8-Pentamethyl-6-Hydroxychroman. A New Dimer, J. Org. Chem. 53, 1281–1284.
Draper, H.H., Csallany, A.S., and Chiu, M. (1966) Isolation of a Trimer of α-Tocopherol from Mammalian Liver, Lipids 2, 47–52.
Suarna, C., and Southwell-Keely, P.T. (1989) Effect of Alcohols on the Oxidation of the Vitamin E Model Compound, 2,2,5,7,8-Pentamethyl-6-chromanol, Lipids 24, 56–60.
Csallany, A.S., and Ha, Y.L. (1992) α-Tocopherol Oxidation Mediated by Superoxide Anion (O2 −). I. Reactions in Aprotic and Protic Conditions, Lipids 27, 195–200.
Ha, Y.L., and Csallany, A.S. (1992) α-Tocopherol Oxidation Mediated by Superoxide Anion (O2 −). II. Identification of the Stable α-Tocopherol Oxidation Products, Lipids 27, 201–205.
Nelan, D.R., and Robeson, C.D. (1962) The Oxidation Product from α-Tocopherol and Potassium Ferricyanide and Its Reaction with Ascorbic and Hydrochloric Acids, J. Am. Chem. Soc. 84, 2963–2965.
Liebler, D.C., Burr, J.A., Matsumoto, S., and Matsuo, M. (1993) Reactions of the Vitamin E Model Compound 2,2,5,7,8-Pentamethylchroman-6-ol with Peroxyl Radicals, Chem. Res. Toxicol. 6, 351–355.
Liebler, D.C., Burr, J.A., Philips, L., and Ham, A.J.L. (1996) Gas Chromatography—Mass Spectrometry Analysis of Vitamin E and Its Oxidation Products, Anal. Biochem. 236, 27–34.
Liebler, D.C., and Burr, J.A. (1992) Oxidation of Vitamin E During Iron-Catalyzed Lipid Peroxidation: Evidence for Electron-Transfer Reactions of the Tocopheroxyl Radical, Biochemistry 31, 8278–8284.
Matsuo, M., Matsumoto, S., Iitaka, Y., and Niki, E. (1989) Radical Scavenging Reactions of Vitamin E and Its Model Compound, 2,2,5,7,8-Pentamethylchroman-6-ol in a tert-Butylperoxyl Radical Generating System, J. Am. Chem. Soc. 111, 7179–7185.
Suarna, C., Baca, M., and Southwell-Keely, P.T. (1992) Oxidation of the α-Tocopherol Model Compound 2,2,5,7,8-Pentamethyl-6-chromanol in the Presence of Alcohols, Lipids 27, 447–453.
Yamauchi, R., Kato, K., and Ueno, Y. (1988) Formation of Trimers of α-Tocopherol and Its Model Compound, 2,2,5,7,8-Pentamethylchroman-6-ol, in Autoxidizing Methyl Linoleate, Lipids 23, 779–783.
Barltrop, J.A., and Coyle, J.D. (1975) Excited States in Organic Chemistry, pp. 49–51, J.D. Wiley & Sons, London.
Rentel, C., Strohschein, S., Albert, K., and Bayer, E. (1998) Silver-Plated Vitamins: A Method of Detecting Tocopherols and Carotenoids in LC/ESI-MS Coupling, Anal. Chem. 70, 4394–4400.
Bayer, E., Gfrörer, P., and Rentel, C. (1999) Coordination-Ion-spray-MS (CIS-MS), a Universal Detection and Characterization Method for Direct Coupling with Separation Techniques, Angew. Chem. Int. Ed. 38, 992–995.
Sauer, J. (1967) Diels-Alder Reactions II(**): The Reaction Mechanism, Angew. Chem. Int. Ed. 6, 16–33.
Seltzer, S. (1965) The Mechanism of the Diels-Alder Reaction of 2-Methylfuran with Maleic Anhydride, J. Am. Chem. Soc. 87, 1534–1540.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Krol, E.S., Escalante, D.D.J. & Liebler, D.C. Mechanisms of dimer and trimer formation from ultraviolet-irradiated α-tocopherol. Lipids 36, 49–55 (2001). https://doi.org/10.1007/s11745-001-0667-y
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-001-0667-y