Abstract
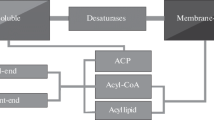
Plant tissues expressing a mammalian stearoyl-CoA Δ9 desaturase were reported to accumulate Δ9 hexadecenoic acid (16∶1), normally very minor in most plant tissues. The transgenic plants were thoroughly analyzed for alterations of individual lipids in different subcellular sites. Western blot analysis indicated that the animal desaturase was targeted to the microsomes. The Δ9 16∶1 was incorporated into both the sn-1 and sn-2 positions of all the major membrane lipids tested, indicating that the endoplasmic reticulum acyltransferases do not exclude unsaturated C16 fatty acids from the sn-2 position. In addition to increases in monounsaturated and decreases in saturated fatty acids, accumulation of 16∶1 was accompanied by a reduction in 18∶3 in all the lipids tested except phosphatidylglycerol, and increases in 18∶2 in phospholipids. Total C16 fatty acid content in the galactolipids of the transgenics was significantly higher than that in the control, but those in the phospholipids were unchanged. In transgenics, Δ11 18∶1 was detected in the sn-1 position of the lipids tested except phosphatidylinositol and phosphatidylserine. Introduction of the animal desaturase, controlled by a seed-specific phaseolin promoter, into soybean somatic embryo resulted in a significant reduction in saturated fatty acids. Such effects were greater in cotyledons than hypocotyl-radicles. This study demonstrated that the animal desaturase can be used to decrease the levels of saturated fatty acids in a crop plant.
Similar content being viewed by others
Abbreviations
- ACP:
-
acyl carrier protein
- BHT:
-
butylated hydroxytoluene
- C n fatty acid(s):
-
fatty acid(s) with n carbon atoms
- DGD:
-
digalactosyldiacylglycerol
- ER:
-
endoplasmic reticulum
- GC:
-
gas chromatography
- KAS:
-
3-ketoacyl-ACP synthase
- LPAAT:
-
lysophosphatidic acid acyltransferase
- MGD:
-
monogalactosyldiacylglycerol
- MS:
-
mass spectrometry
- PA:
-
phosphatidic acid
- PC:
-
phosphatidylcholine
- PE:
-
phosphatidylethanolamine
- PG:
-
phosphatidylglycerol
- PI:
-
phosphatidylinositol
- PS:
-
phosphatidylserine
- RDS:
-
rat liver stearoyl-CoA Δ9 desaturase
- SDS:
-
sodium dodecyl sulfate
- X/Y:
-
a fatty acyl group containing X carbon atoms and Y cis double bonds
- UV:
-
ultraviolet
References
Somerville, S., and Browse, J. (1991) Plant Lipids: Metabolism, Mutants, and Membranes, Science 252, 80–87.
Schmidt, H., and Heinz, E. (1990) Involvement of Ferredoxin in Desaturation of Lipid-Bound Oleate in Chloroplasts, Plant Physiol. 94, 214–220.
Smith, M.A., Cross, A.R., Jones, O.T.G., Griffiths, W.T., Stymne, S., and Stobart, K. (1990) Electron-Transport Components of the 1-Acyl-2-oleoyl-sn-glycero-3-phosphocholine Δ12-Desaturase (Δ12-desaturase) in Microsomal Preparations from Developing Safflower (Carthamus tinctorius L.) Cotyledons, Biochem. J. 272, 23–29.
Kearns, E.V., Hugly, S., and Somerville, C. (1991) The Role of Cytochrome b5 in Δ12 Desaturation of Oleic Acid by Microsomes of Safflower (Carthamus tinctorius L.), Arch. Biochem. Biophys. 284, 431–436.
Browse, J., and Somerville, C. (1991) Glycerolipid Synthesis: Biochemistry and Regulation, Annu. Rev. Plant. Physiol. 42, 467–506.
Frentzen, M., Heinz, E., Mckeon, T.A., and Stumpf, P.K. (1983) Specificities and Selectivities of Glycerol-3-phosphate Acyltransferase and Monoacylglycerol-3-phosphate Acyltransferase from Pea and Spinach Chloroplasts, Eur. J. Biochem. 129, 629–636.
Frentzen, M. (1993) Acyltransferases and Triacylglycerols, in Lipid Metabolism in Plants (Moore, T.S., Jr., ed.) pp. 195–231, CRC Press, Boca Raton.
Polashock, J.J., Chin, C.-K., and Martin, C.E. (1992) Expression of the Yeast Δ-9 Fatty Acid Desaturase in Nicotiana tabacum, Plant Physiol. 100, 894–901.
Ohlrogge, J., and Browse, J. (1995) Lipid Biosynthesis, Plant Cell 7, 957–970.
Schnebly, S.R., and Fehr, W.R. (1993) Effect of Years and Planting Dates on Fatty Acid Composition of Soybean Genotypes, Crop Sci. 33, 716–719.
Sizer, F., and Whitney, E. (1994) Nutrition Concepts and Controversies, 6th edn., West Publishing Co., St. Paul, MN.
Grayburn, W.S., Collins, G.B., and Hildebrand, D.F. (1992) Fatty Acid Alteration by a Δ9 Desaturase in Transgenic Tobacco Tissue, Biotechnology 10, 675–678.
Liu, W., Torisky, R.S., McAllister, K.P., Avdiushko, S., Hildebrand, D., and Collins, G.B. (1996) Somatic Embryo Cycling: Evaluation of a Novel Transformation and Assay System for Seed-Specific Gene Expression in Soybean, Plant Cell Tissue Organ Cult. 47, 33–42.
Liu, W., Moore, P.J., and Collins, G.B. (1992) Somatic Embryogenesis in Soybean via Somatic Embryo Cycling, In Vitro Cell Dev. Biol. 28P, 153–160.
Dahmer, M.L., Fleming, P.D., Collins, G.B., and Hildebrand, D.F. (1989) A Rapid Screening Technique for Determining the Lipid Composition of Soybean Seeds, J. Am. Oil Chem. Soc. 66, 543–548.
Miquel, M., and Browse, J. (1992) Arabidopsis Mutants Deficient in Polyunsaturated Fatty Acid Synthesis: Biochemical and Genetic Characterization of a Plant Oleoyl-Phosphatidylcholine Desaturase, J. Biol. Chem. 267, 1502–1509.
Khan, M.-U., and Williams, J.P. (1977) Improved Thin-Layer Chromatographic Method for the Separation of Major Phospholipids and Glycerolipids from Plant Lipid Extracts and Phosphatidylglycerol and bis (monoacylglyceryl) Phosphate from Animal Lipid Extracts, J. Chromatogr. 140, 179–185.
Yamamoto, K., Shibahara, A., Nakayama, T., and Kajimoto, G. (1991) Determination of Double-Bond Position in Methylene-Interrupted Dienoic Fatty Acids by GC-MS as Their Dimethyl Disulfide Adducts, Chem. Phys. Lipids 60, 39–50.
Fischer, W., Heinz, E., and Zeus, M. (1973) The Suitability of Lipase from Rhizopus arrhizus delemar for Analysis of Fatty Acid Distribution in Dihexosyl Diglycerides, Phospholipids and Plant Sulfolipids, Hoppe-Seyler's Z. Physiol. Chem. 354, 1115–1123.
Lord, J.M., Kagawa, T., and Beevers, H. (1972) Intracellular Distribution of Enzymes of the Cytidine Diphosphate Choline Pathway in Castor Bean Endosperm, Proc. Natl. Acad. Sci. USA 69, 2429–2432.
Roughan, P.G., Holland, R., and Slack, C.R. (1980) The Role of Chloroplasts and Microsomal Fractions in Polar-Lipid Synthesis from [1-14C]Acetate by Cell-Free Preparations from Spinach (Spinacia oleracea) Leaves, Biochem. J. 188, 17–24.
Tolbert, N.E. (1974) Isolation of Subcellular Organelles of Metabolism on Isopycnic Sucrose Gradients, Methods Enzymol. 31, 734–746.
Arnon, D.I. (1947) Copper Enzymes in Isolated Chloroplasts: Polyphenoloxidase in Beta vulgaris, Plant Physiol. 24, 1–15.
Grayburn, W.S., and Hildebrand, D.F. (1995) Progeny Analysis of Tobacco That Express a Mammalian Δ9 Desaturase, J. Am. Oil Chem. Soc. 72, 317–321.
Holloway, P.W. (1983) Fatty Acid Desaturation, in The Enzymes (Boyer, P.D., ed.) Vol. 16, pp. 63–83, Academic, New York.
Browse, J., Warwick, N., Somerville, C.R., and Slack, C.R. (1986) Fluxes Through the Prokaryotic and Eukaryotic Pathways of Lipid Synthesis in the “16∶3” Plant Arabidopsis thaliana, Biochem. J. 235, 25–31.
Roughan, P.G., and Slack, C.R. (1982) Cellular Organization of Glycerolipid Metabolism, Annu. Rev. Plant Physiol. 33, 97–132.
Nishida, I., and Murata, N. (1996) Chilling Sensitivity in Plants and Cyanobacteria: The Crucial Contribution of Membrane Lipids, Annu. Rev. Plant Physiol. 47, 541–546.
Enoch, H.G., Catala, A., and Strittmatter, P. (1976) Mechanism of Rat Liver Microsomal Stearyl-CoA Desaturase: Studies of the Substrate Specificity, Enzyme-Substrate Interactions, and the Function of Lipid, J. Biol. Chem. 251, 5095–5103.
Mckeon, T.A., and Stumpf, P.K. (1982) Purification and Characterization of the Stearoyl-Acyl Carrier Protein Desaturase and the Acyl-Acyl Carrier Protein Thioesterase from Maturing Seeds of Safflower, J. Biol. Chem. 257, 12141–12147.
Williamson, J.D., Hirsch-Wyncott, M.E., Larkins, B.A., and Gelvin, S.B. (1989) Differential Accumulation of a Transcript Driven by the CaMV 35S Promoter in Transgenic Tobacco, Plant Physiol. 90, 1570–1576.
Shibahara, A., Yamamoto, K., Takeoka, M., Kinoshita, A., Kajimoto, G., Nakayama, T., and Noda, M. (1989) Application of a GC-MS Method Using Deuterated Fatty Acids for Tracing cis-Vaccenic Acid Biosynthesis in Kaki Pulp, Lipids24, 488–493.
Shibahara, A., Yamamoto, K., Takeoka, M., Kinoshita, A., Kajimoto, G., Nakayama, T., and Noda, M. (1990) Novel Pathways of Oleic and cis-Vaccenic Acid Biosynthesis by an Enzymatic Double-Bond Shifting Reaction in Higher Plants, FEBS Lett. 264, 228–230.
Ichihara, K., Asahi, T., and Fujii, S. (1987) 1-Acyl-sn-glycerol-3-phosπphate Acyltransferase in Maturing Safflower Seeds and Its Contribution to the Non-Random Fatty Acid Distribution of Triacylglycerol, Eur. J. Biochem. 167, 339–347.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Moon, H., Hazebroek, J. & Hildebrand, D.F. Changes in fatty acid composition in plant tissues expressing a mammalian Δ9 desaturase. Lipids 35, 471–479 (2000). https://doi.org/10.1007/s11745-000-546-6
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-000-546-6