Abstract
The antibacterial activity of alanine-derived gemini quaternary ammonium salts (chlorides and bromides) with various spacer and alkyl chain lengths was investigated. The studied compounds exhibited a strong bactericidal effect, especially bromides with 10 and 12 carbon alkyl chains and 3 carbon spacer groups (TMPAL-10 Br and TMPAL-12 Br), with a short contact time. Both salts dislodged biofilms of Pseudomonas aeruginosa and Staphylococcus epidermidis, and were lethal to adherent cells of S. epidermidis. Bromide with 2 carbon spacer groups and 12 carbon alkyl chains (TMEAL-12 Br) effectively reduced microbial adhesion by coating polystyrene and silicone surfaces. The results obtained suggest that, after further studies, gemini QAS might be considered as antimicrobial agents in medicine or industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quaternary ammonium salts (QAS) are commonly used in medicine and industry. These cationic surfactants are applied as preservatives, biocides, disinfectants and muscle relaxants [1, 2]. Gemini quaternary ammonium salts (gemini QAS) are built of two monomeric QAS molecules linked by a spacer. Their good surface activity is due to the presence of two hydrophilic head groups and two hydrophobic alkyl chains. Gemini QAS have much lower CMC (critical micelle concentrations) in comparison to monomeric surfactants [3]. Gemini QAS surfactants are able to form bilayer aggregates, like liposomes, and are extensively studied as potential non-viral gene delivery systems or drug carriers [4–6]. The activity of gemini QAS against microorganisms is generally stronger in comparison to the corresponding monomeric compounds and depends on the structure of the gemini molecule [7].
Previous research regarding gemini QAS with betaine ester type arrangements showed that low concentrations of these compounds inhibited bacterial and fungal growth. This activity depended on the alkyl chain lengths, spacer structure and the counterion, with the greatest growth reduction being exhibited by N,N′-bis[2-dodecyloxy-2-oxoethyl]-N,N,N′,N′-tetramethylethane-1,2-diammonium dichloride. The betaine ester gemini surfactants strongly affected bacterial and fungal biofilms (i.e., multicellular communities surrounded by extracellular polymeric substances). Moreover, these compounds inhibited cell adhesion and prevented biofilm formation by coating the surface. Strong anti-biofilm activity was observed especially against Pseudomonas aeruginosa and Staphylococcus epidermidis biofilms [8, 9]. These two species are common contaminants of hospital environment and a frequent cause of nosocomial infections. P. aeruginosa is especially dangerous for cystic fibrosis (CF) patients, who are vulnerable to lung infections. The formation of P. aeruginosa biofilms is promoted by many determinants (e.g., fimbriae, proteins, eDNA). Biofilm cells are surrounded by a polysaccharide matrix that plays an important role in biofilm maintenance and resistance to antibiotics [10, 11].
S. epidermidis colonizes human skin and mucosal membranes. It is also a common contamination of medical devices (e.g., catheters) and a cause of bacteremia, mainly in immunocompromised patients and neonates. The virulence of S. epidermidis is often associated with the ability of this species to form biofilms on polymeric surfaces. Intercellular adhesion and biofilm accumulation is mediated by several factors, such as PIA (polysaccharide intercellular adhesion), Aap (accumulation associated protein) or Embp (extracellular matrix binding protein) [12, 13].
Biofilms are extremely hard to eliminate. Adherent cells usually exhibit antibiotic tolerance due to the altered metabolism and components of the biofilm matrix. Polymeric extracellular matrix, enriched in eDNA and proteins, makes the biofilm structure robust and resistant to eradication, and thus there is a need for new compounds with good anti-biofilm properties [14, 15].
To investigate whether the antimicrobial activity depends on the head group structure, a series of gemini QAS (with different alkyl chain and spacer lengths) that mimic alanine was synthesized [16]. In the present study we investigate their biological activity against Gram-positive and Gram-negative bacteria as well as anti-adhesive and biofilm dislodging capacities.
Experimental Methods
Chemicals
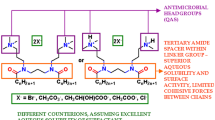
The structure of the alanine-derived gemini quaternary ammonium salts (QAS), synthesized at the Department of Chemistry, Technical University of Wroclaw, Poland, as described previously [16] is shown in Fig. 1.
Structure of tested gemini quaternary ammonium, derivatives of N,N,N′,N′-tetramethylethylenediamine: TMEAL-n Br (N,N-bis(1-(n-alkyloxy)-1-oxopronan-2-yl)-N,N,N′,N′-tetramethylethane-1,2-diammonium dibromide); TMEAL-n Cl (N,N′-bis(1-(n-alkyloxy)-1-oxopronan-2-yl)-N,N,N′,N′-tetramethylethane-1,2-diammonium dichloride) or N,N,N′,N′-tetramethyl-1,3-propanediamine: TMPAL-n Br N,N′-bis(1-(n-alkyloxy)-1-oxopronan-2-yl)-N,N,N′,N′-tetramethylpropane-1,3-diammonium dibromide; TMPAL-n Cl (N,N′-bis(1-(n-alkyloxy)-1-oxopronan-2-yl)-N,N,N′,N′-tetramethylpropane-1,3-diammonium dichloride)
Strains
In the present study we used the following clinical strains: Staphylococcus epidermidis B374 MRSE, Enterococcus faecalis 30VRE (resistant to vancomycin) from Wroclaw Medical University as well as reference strains: Staphylococcus aureus ATCC 6538, Escherichia coli ATCC 11229 and Pseudomonas aeruginosa PAO1 from Institute of Genetics and Microbiology, University of Wrocław collection.
Evaluation of Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC)
Minimal inhibitory concentrations of the studied gemini QAS (at a concentration range of 1–800 µM) against bacterial strains were determined using the broth dilution method recommended by NCCLS, M7-A5 [17]. Strains were incubated with or without (growth control) gemini QAS compounds for 24 h at 37 °C. Each sample was run in duplicate. The MIC values were determined spectrophotometrically and expressed as the concentration of the gemini surfactant that completely inhibited bacterial growth. Optical density of each well was measured at λ550 using an 96-well microplate reader (Asys Hitachi 340, Driver Version: 4.02, Biogenet, Poland). The experiment was repeated three times.
To determine the minimal bactericidal concentration (MBC) 100 µl of bacterial suspension incubated with gemini QAS at the MIC, 2 × MIC and 4 × MIC was transferred to Luria Broth (1 % tryptone, 1 % yeast extract, 0.5 % NaCl) plate. MBC were expressed as the concentration of the gemini surfactant that reduced the number of colony forming units (cfu) by 99.9 % after 24 h of incubation at 37 °C, as described elsewhere [18].
Short-Time Killing Assay
Short-time killing assay was undertaken using S. epidermidis B374 strain to determine bactericidal activity of gemini QAS. Bacterial cultures were suspended in physiologic salt solution overnight and turbidity was adjusted to the 0.5 standards of the McFarland scale. Suspensions were then diluted to obtain 104 cells/ml in LB medium. Gemini surfactants were added to the bacterial suspensions to obtain a final concentration equal to the MBC. Cells were incubated at 37 °C with constant agitation (250 rpm). At each time point (0, 5, 15, 30, 60 and 120 min) samples (10 µl) were transferred onto LB agar plates. Plates were incubated at 37 °C for 24 h and colonies were counted.
Adhesion Inhibition
The reduction of bacterial adhesion to polystyrene and silicone surfaces was determined according to Cremet et al. and Silva, respectively [19, 20]. Briefly, a 96-well polystyrene microtiter plate or 2 cm fragments of silicone catheters were incubated with various concentrations of gemini QAS for 2 h at 37 °C and washed with distilled water. A sample (100 µl) of P. aeruginosa PAO1 or S. epidermidis suspended in LB (OD 0.6) was added and the surfaces were incubated for an additional 4 h. After 5-min staining with crystal violet, absorbance was measured at λ590 using an Asys Hitachi 340 instrument (Biogenet, Poland).
Biofilm Viability
P. aeruginosa and S. epidermidis biofilm viability were tested with a FilmTracer LIVE/DEAD BacLight Biofilm Viability Kit (Invitrogen) according to Obłąk [8]. Biofilms were grown on glass chamber slides in LB medium for 24 h, at 37 °C and washed with distilled water. Gemini surfactants: TMPAL-10 Br, TMPAL-12 Br and TMEAL-12 Br were applied at a concentration of 120 µM and biofilms were incubated for 2 h. The compounds were removed and biofilms were stained with LIVE/DEAD fluorescent dye. For microscopic observations an Olympus BX51 fluorescence microscope was used.
Statistical Analysis
To estimate the significance of the impact of gemini QAS on bacterial growth and adhesion, the statistical analysis tests were performed using Statistica 12. All experiments were repeated at least three times and the significance was stated at a p value <0.05.
Results
Minimal Inhibitory Concentration (MIC)
The evaluation of minimal inhibitory concentrations allowed us to determine the activity of gemini quaternary ammonium salts with various lengths of alkyl chains (C6–C14) and spacer ((CH2)2 or (CH2)3) against Gram-positive (S. epidermidis, S. aureus and E. faecalis) and Gram-negative bacteria (P. aeruginosa).
It was shown that the molecular structure of the surfactant had an influence on its antibacterial activity—generally the compounds with 12 carbon atoms exhibited stronger antibacterial activity (p = 0.0005). Similarly, gemini QAS with longer spacers (three methylene groups) were more effective in comparison to compounds with two methylene groups within the spacer (p = 0.0002) (Table 1).
Comparison between the antibacterial effect against Gram-positive and Gram-negative bacteria showed that the impact of gemini QAS on bacterial growth depended on the strain. Generally, two tested strains of staphylococci were rather sensitive to gemini surfactants, whereas vancomycin-resistant E. faecalis and P. aeruginosa PAO1 exhibited higher tolerance (p < 0.04).
It was also shown that there is a correlation between the counterion of a gemini QAS and its antibacterial activity, since the bromides had a stronger effect on bacterial growth than the chlorides (p = 0.038).
The strongest bactericidal activity against both Gram-positive and Gram-negative bacteria was exhibited by surfactants with longer spacers and C10–C12 alkyl chains—TMPAL-10 Br and TMPAL-12 Br (MBC of 20–80 µM). Low MIC values against Gram-positive strains were also observed for the compound with a shorter spacer and 12 carbon atoms (TMEAL-12 Br), but the minimal bactericidal concentrations were much higher, indicating a rather bacteriostatic effect of this compound (Tables 1, 2).
Short-Time Killing
The short-time killing was investigated using the two most active gemini QAS (TMPAL-10 Br and TMPAL-12 Br) against S. epidermidis B374 (Table 2). It was shown that these two surfactants already exhibited a lethal effect against S. epidermidis cells after 5 min (0.15 % survival) and reached 100 % lethality after 60–120 min (Table 3). There were also some significant differences in the effect on cell survival between these two compounds. After 5-min contact TMPAL-10 Br is more lethal towards bacterial cells (p = 0.0408), however additional incubation up to 60 min showed greater impact of TMPAL-12 Br on the survival (p = 0.0065).
Adhesion
The adhesion of bacterial cells to the surface is the first stage of biofilm development. The gemini quaternary ammonium salts may be able to coat the surface and prevent cell adhesion. The research on adhesion inhibition showed that only the compounds with 12 carbon atoms within alkyl chains reduced S. epidermidis adhesion to a polystyrene plate. A greater inhibition was displayed by TMEAL-12 Br, which reduced S. epidermidis adhesion by about 50 % at a low concentration (20 µM), but significant reduction was observed already at 10 µM (p = 0.0035). On the other hand, P. aeruginosa adhesion to polystyrene was not inhibited to this extent by any of the compounds tested. However, TMEAL-12 Br showed a significant reduction in bacterial adhesion (by about 20 %) at 120 µM (p = 0.01) (see Fig. 2). TMEAL-12 Br was also effective in coating silicone catheters, since it significantly reduced the adhesion of both S. epidermidis (p = 0.017) and P. aeruginosa (p = 0.038), whereas TMPAL-12 Br did not show any significant anti-adhesive activity (Fig. 3).
Biofilm Viability
Staining of bacterial biofilms with SYTO9/propidium iodide showed that P. aeruginosa PAO1 biofilm was eradicated by TMPAL-10 Br and TMPAL-12 Br. The amount of observed adherent cells was lower in comparison to the control, however the remaining biofilm was viable (green fluorescence) (Fig. 4a, c, e). S. epidermidis biofilm, on the other hand, was much more sensitive to gemini QAS. Both TMPAL-10 Br and TMPAL-12 Br caused a large drop in biofilm viability, manifested by the red fluorescence of propidium iodide (Fig. 4b, d, f). TMEAL-12 Br did not exhibit any significant effect on P. aeruginosa and S. epidermidis biofilms (Fig. 4g, h).
Discussion
Gemini quaternary ammonium salts are a class of surfactants built of two monomeric QAS molecules linked by a spacer [21]. They exhibit stronger surface and biological activity in comparison to conventional QAS, which are widely used as drugs and disinfectants [22–24]. Due to the overuse of cleaning agents, there is a problem of increasing microbial cross-resistance, that could be overcome by the development of new antimicrobial compounds [1].
Gemini QAS show strong antibacterial and antifungal activity [25, 26]. Betaine- and alanine-derived gemini surfactants did not show cytotoxic effects towards yeast mitochondrial metabolism and they were not mutagenic [9, 27]. Previously investigated gemini QAS, having betaine-based, ester-type alkyl chain arrangements, inhibited S. aureus growth at low concentrations (10–80 µM). Chlorides with 10 and 12 carbon alkyl chains were also effective in eradicating P. aeruginosa [8]. Compared with betaine QAS surfactants, alanine derivatives with shorter spacer show weaker antibacterial activity both against Gram-positive and Gram-negative bacteria. On the other hand, elongation of the spacer increased biological activity to a higher level than in the case of betaine QAS gemini surfactants.
The strongest antibacterial effect was exhibited by bromides with longer spacers and alkyl chains of 10 or 12 carbons (TMPAL-10 Br and TMPAL-12 Br). These compounds inhibited growth of Gram-positive and Gram-negative strains at low concentrations and, more importantly, they were lethal to S. epidermidis after a short time of exposure. It has been shown previously that the biological activity of gemini surfactants depends on the spacer length. When the distance between alkyl chains was larger, the incorporation of surfactant into the plasma membrane of erythrocytes was easier and, in consequence, an increased level of cell disruption was observed [16].
The compounds with a shorter spacer (TMEAL-10 Br, TMEAL-12 Br) showed good antibacterial activity, but only against Gram-positive strains, since P. aeruginosa and E. coli exhibited tolerance to higher concentrations of these gemini QAS. A similar effect was observed for betaine QAS gemini surfactants [8]. The differences in gemini surfactant tolerance between Gram-positive and Gram-negative bacteria might be connected with the cell envelope structure. The presence of LPS molecules, outer membrane proteins or numerous efflux systems in P. aeruginosa and E. coli might be responsible for the resistance to gemini QAS [28, 29].
Many bacterial strains are able to live either as planktonic forms or in the biofilm structure. Biofilms are multicellular bacterial communities composed of cells surrounded by extracellular polymeric substances (EPS). EPS contains mostly polysaccharides, proteins and nucleic acids and protects the community from changes in environmental conditions. Bacterial biofilms are hard to eradicate due to the increased resistance to antimicrobial agents. The bacterial ability to form biofilms is often a cause of infections associated with medical devises (e.g., catheters) [14, 30]. The biofilm development starts with cell attachment to biotic or abiotic surfaces and this process involves many properties of the cell. Preventing bacterial adhesion is one of the strategies to stop biofilm formation and to counteract bacterial pathogenesis [31]. One of the modes of adhesion inhibition is changing the surface properties by anti-adhesive coatings. The examples include silver, heparin or sparfloxacin coatings of catheters or QAS-containing dental polymers [32–34]. It was previously shown that betaine QAS gemini surfactants with C12 and C14 carbon alkyl chains coat the polystyrene surface and reduce S. epidermidis adhesion and biofilm development [8]. Similarly, alanine-derived QAS gemini surfactants with 12 carbon alkyl chains exhibited anti-adhesive properties on the polystyrene surface at low concentrations, but only against S. epidermidis. Surprisingly, the coating of silicone catheters with TMEAL-12 Br inhibited cell adhesion of both S. epidermidis and P. aeruginosa. These results might suggest that this compound coated silicone more effectively than polystyrene and the amount of gemini QAS molecules deposited on the catheter is enough to block cell adhesion and reduce P. aeruginosa biofilm formation.
Mature bacterial biofilms exhibit increased tolerance to disinfectants and antibiotics. There are numerous determinants for the resistance, e.g., altered metabolism of adherent cells, overexpression of degrading enzymes, active efflux and poor penetration of biofilm structure by drugs [35]. Previously studied betaine-like gemini bromide with 12 carbon alkyl chains showed strong biofilm-dislodging properties against both P. aeruginosa and S. epidermidis [8]. Alanine derivatives with 10 and 12 carbon alkyl chains (TMPAL-10 Br and TMPAL-12 Br) reduced biofilm formation by both these species. What is more, the remaining adherent cells of S. epidermidis were killed after exposure to gemini QAS, since they failed to exclude propidium iodide. On the other hand, P. aeruginosa undislodged biofilm remained viable.
Although overall comparison of betaine- and alanine-derived gemini surfactants indicates that the latter exhibit weaker biological activity, the alanine bromides with longer spacer (TMPAL-n Br) have lower MIC and MBC and might in the future be considered for further studies towards application in medicine and industry.
References
Hegstad K, Langsrud S, Lunestad BT, Scheie AA, Sunde M, Yazdankhah SP (2010) Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health? Microb Drug Resis 16:91–104
Zayed SI (2011) Flow injection potentiometric determination of pancuronium bromide in pharmaceutical preparation and urine samples using modified carbon paste electrodes. Chem Pharm Bull 59:254–259
Hait SK, Moulik SP (2002) Gemini surfactants: a distinct class of self-assembling molecules. Curr Sci 82:1101–1111
Aleandri S, Bombelli C, Bonicelli MG, Bordi F, Giansanti L, Mancini G, Ierino M, Sennato S (2013) Fusion of gemini based cationic liposomes with cell membrane models: implications for their biological activity. Biochim Biophys Acta 1828:382–390
Cosimati R, Milardi GL, Bombelli C, Bonicontro A, Bordi F, Mancini G, Risuleo G (2013) Interactions of DMPC and DMPC/gemini liposomes with the cell membrane investigated by electrorotation. Biochim Biophys Acta 1828:352–356
Munoz-Ubeda M, Misra SK, Barran-Berdon AL, Datta S, Aicart-Ramos C, Castro-Hartmann P, Kondaiah P, Junguera E, Bhattacharya S, Aicart E (2012) How does the spacer length of cationic gemini lipids influence the lipoplex formation with plasmid DNA? Physicochemical and biochemical characterizations and their relevance in gene therapy. Biomacromolecules 13:3926–3937
Perez L, Pinazo A, Pons R, Infante MR (2014) Gemini surfactants from natural amino-acids. Adv Colloid Interface Sci 205:134–155
Obłąk E, Piecuch A, Guz-Regner K, Dworniczek E (2014) Antibacterial activity of gemini quaternary ammonium salts. FEMS Microbiol Lett 350:190–198
Obłąk E, Piecuch A, Krasowska A, Łuczyński J (2013) Antifungal activity of gemini quaternary ammonium salts. Microbiol Res 168:630–638
Mikkelsen H, Sivaneson M, Filloux A (2011) Key two-component regulatory systems that control biofilm formation in Pseudomonas aeruginosa. Environ Microbiol 13:1666–1681
Ryder C, Byrd M, Wozniak DJ (2007) Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr Opin Microbiol 10:644–648
Otto M (2009) Staphylococcus epidermidis: the “accidental” pathogen. Nat Rev Microbiol 7:555–567
Otto M (2012) Molecular basis of Staphylococcus epidermidis infections. Semin Immunopathol 34:201–214
Tan SY, Chew SC, Tan SY, Givskov M, Yang L (2014) Emerging frontiers in detection and control of bacterial biofilms. Curr Opin Biotechnol 26:1–6
Penesyan A, Gillings M, Paulsen IT (2015) Antibiotic discovery: combatting bacterial resistance in cells and in biofilm communities. Molecules 24:5286–5298
Łuczyński J, Frąckowiak R, Włoch A, Kleszczyńska H, Witek S (2013) Gemini esterquat surfactants and their biological activity. Cell Mol Biol Lett 18:89–101
Nationale Committee for Clinical Laboratory Standards (2000) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved Standard-fifth edition, NCCLS document 20(2): M7-A5
Begec Z, Yucel A, Yakupogullari Y, Erdogan MA, Duman Y, Durmus M, Ersoy MO (2013) The antimicrobial effects of ketamine combined with propofol: an in vitro study. Rev Bras Anestesiol 63:461–465
Cremet L, Corvec S, Batard E, Auger M, Lopez I, Pagniez F, Dauvergne S, Caroff N (2013) Comparison of three methods to study biofilm formation by clinical strains of Escherichia coli. Diagn Microbiol Infect Dis 75:252–255
Silva S, Negri M, Henriques M, Oliveira R, Williams D, Azeredo J (2010) Silicone colonization by non-Candida albicans Candida species in the presence of urine. J Med Microbiol 59:747–754
Shukla D, Tyagi VK (2006) Cationic gemini surfactants: a review. J Oleo Sci 55:381–390
Sakai K, Umemoto N, Aburai K, Takamatsu Y, Endo T, Kitiyanan B, Matsumoto M, Sakai H, Abe M (2014) Physicochemical properties of oleic acid-based partially fluorinated gemini surfactants. J Oleo Sci 63:257–267
Tatsumi T, Imai Y, Kawaguchi K, Miyano N, Ikeda I (2014) Antimicrobial activity of cationic gemini surfactant containing an oxycarbonyl group in the lipophilic portion against gram-positive and gram-negative microorganisms. J Oleo Sci 63:137–140
Zhang Q, Gao Z, Xu F, Tai S, Liu X, Mo S, Niu F (2012) Surface tension and aggregation properties of novel cationic gemini surfactants with diethylammonium head groups and a diamido spacer. Langmuir 28:11979–11987
Shirai A, Sumitomo T, Yoshida M, Kaimura T, Nagamune H, Maeda T, Kourai H (2006) Synthesis and biological properties of gemini quaternary ammonium compounds, 5,5-[2,2-(alpha, omega-polymethylnedicarbonyldioxy)diethyl]bis-(3-alkyl-4-methylthiazolium iodide) and 5,5-[2,2-(p-phenylenedicarbonyl-dioxy)diethyl]bis(3-alkyl-4-methylthiazolium bromide). Chem Pharm Bull 54:639–645
Sumitomo T, Maeda T, Nagamune H, Kourai H (2004) Bacterioclastic action of a bis-quaternary ammonium compound against Escherichia coli. Biocontrol Sci 8:145–149
Obłąk E, Piecuch A, Dworniczek E, Olejniczak T (2015) The influence of biodegradable gemini surfactants, N,N′-bis(1-decyloxy-1-oxopronan-2-yl)-N,N,N′,N′tetramethyl- propane-1,3-diammonium dibromide and N,N′-bis(1-dodecyloxy-1-oxopronan-2-yl) N,N,N′,N′-tetramethylethane-1,2-diammonium dibromide, on fungal biofilm and adhesion. J Oleo Sci 64:527–537
McDonnell G, Russell AD (1999) Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev 12:147–179
Poole K (2002) Mechanisms of bacterial biocide and antibiotic resistance. J Appl Microbiol 92(Suppl):55S–64S
Tran N, Tran PA (2012) Nanomaterial-based treatments for medical device-associated infections. ChemPhysChem 13:2481–2494
Klemm P, Vejborg RM, Hancock V (2010) Prevention of bacterial adhesion. Appl Microbiol Biotechnol 88:451–459
Kowalczuk D, Ginalska G, Golus J (2010) Characterization of the developed antimicrobial urological catheter. Int J Pharm 402:175–183
Li F, Weir MD, Chen J, Xu HHK (2013) Comparison of quaternary ammonium-containing with nanosilver-containing adhesive in antibacterial properties and cytotoxicity. Dent Mater 29:450–461
Zhang K, Melo MAS, Cheng L, Weir MD, Bai Y, Xu HHK (2012) Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dent Mater 28:842–852
Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O (2010) Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35:322–332
Acknowledgments
The work was supported by the Polish Ministry of Science and Higher Education grants No N N303 0685 34 and No N N209 337737. Gemini-QAS surfactants were synthesized by Dr. Jacek Łuczyński at Wrocław University of Technology. We are sincerely grateful to Dr Ewa Dworniczek from the Department of Microbiology at Wroclaw Medical University for her kind help with the fluorescence studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Piecuch, A., Obłąk, E. & Guz-Regner, K. Antibacterial Activity of Alanine-Derived Gemini Quaternary Ammonium Compounds. J Surfact Deterg 19, 275–282 (2016). https://doi.org/10.1007/s11743-015-1778-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-015-1778-3