Abstract
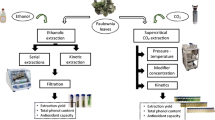
Juá (Ziziphus joazeiro) is a Brazilian plant and its bark has been used as a detergent and phytotherapic due to its high saponin content (2–10 %). Saponins are triterpenic glycosides with some properties that aid their use in food, cosmetic and pharmaceutical industries. The object of the present work was to develop an extraction and concentration process of saponins from jua bark, using green solvents such as water and ethanol. Firstly, the extraction conditions optimization was carried out using a central composite design, and compared with other methods such as Soxhlet, ultrasound-assisted extraction and micellar extraction. Then, cloud point preconcentration was tested to select the salt type and its concentration which promotes a higher concentration factor and partition coefficient at room temperature. Finally, the removal of a t-octyl phenol ethoxylate (9–10 EO) nonionic surfactant by adsorption was evaluated by optimizing the adsorbent type and its concentration, temperature and time of adsorption, in addition to the adsorbent recycling. Orbital shaker extraction leads to a recovery of 45.6 % saponins under the following conditions: temperature, 38.8 °C; jua/solvent ratio, 0.272; stirring speed, 300 rpm; extraction time, 2 h. Under these conditions, saponins recovery reached 90.8 % when using 15 % v/v of the nonionic surfactant, and a preconcentration factor of 14.2 was obtained by adding sodium carbonate 20 % w/v. The preconcentration factor decreased to a value of 10.1, after nonionic surfactant removal by a hydrophobic crosslinked polystyrene copolymer resin.








Similar content being viewed by others
References
Barbosa-Filho JM, Trigueiro JA, Cheriyan U, Bhattacharyya J (1985) Constituents of stem-bark of Zizyphus joazeiro. J Nat Prod 48:152–153
Barbosa-Filho JM (1997) Quimiodiversidade e potencialidade farmacológica da flora paraibana. Cad Farmácia 13:85–102
Higuchi R, Kubota S, Komori T, Kawasaki T, Pandey VB, Singh JP, Shah AH (1984) Triterpenoid saponins from the bark of Zizyphus joazeiro. Phytochem 23:2597–2600
Schühly W, Heilmann J, Çalis I, Sticher O (2000) Novel Triterpene Saponins from Zizyphus joazeiro. Helv Chim Acta 83:1509–1516
Güçlü-Üstündag Ö, Mazza G (2007) Saponins: properties, applications and processing. Crit Rev Food Sci Nutr 47:231–258
Kalinowska M, Zimowski J, Paczkowski C, Wojciechowski ZA (2005) The formation of sugar chains in triterpenoid saponins and glycoalkaloids. Phytochem Rev 4:237–257
Oleszek WA (2000) Saponins. In: Naidu AS (ed) Natural food antimicrobial systems, CRC Press, Boca Raton
Sparg SG, Light ME, Van Staden J (2004) Biological activities and distribution of plant saponins. J Ethnopharm 94:219–243
Vincken JP, Heng L, De Groot A, Gruppen H (2007) Saponins, classification and occurrence in plant kingdom. Phytochem 68:275–297
Gafner S, Bergeron C, McCollom MM, Cooper LM, McPhail KL, Gerwick WH, Angerhofer CK (2004) Evaluation of the efficiency of three different solvent systems to extract triterpene saponins from roots of Panax quinquefolius using High-Performance Liquid Chromatography. J Agric Food Chem 52:1546–1550
Fang Q, Yeung HW, Leung HW, Huie CW (2000) Micelle-mediated extraction and preconcentration of ginsenosides from Chinese herbal medicine. J Chromat A 904:47–55
Wanezaki S, Tsuzaki S, Araki H (2005) Soybean saponin-containing material and process for producing the same. US2005/0123662 A1
Wang Q, Ma S, Fu B, Lee FSC, Wang X (2004) Development of multi-stage countercurrent extraction technology for the extraction of glycyrrhizic acid (GA) from licorice (Glycyrrhiza uralensis Fisch). Biochem Eng J 21:285–292
Zhang SQ, Zhang JS, Wang CZ (2007) Extraction of steroid saponins from Paris polyphylla Sm. var. yunnanensis using novel ultrahigh pressure extraction technology. Pharm Chem J 41:27-3
Ong ES (2004) Extraction methods and chemical standardization of botanicals and herbal preparations. J Chromat B 812:23–33
Wang L, Weller CL (2006) Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol 17:300–312
Ong ES, Len SM (2003) Pressurized hot water extraction of berberine, baicalein and glycyrrhizin in medicinal plants. Anal Chim Acta 482(1):81–89
Zhao S, Kwok KC, Liang H (2007) Investigation on ultrasound assisted extraction of saikosaponins from Radix Bupleuri. Sep Purif Technol 55:307–312
Makkar HPS, Siddhuraju P, Becker K (2007) Methods in molecular biology. In: Plant Secondary Metabolites. vol 393 Humana Press, Totowa
Waterman PG, Mole S (1994) Analysis of phenolic plant metabolites. Blackwell Scientific Publications, Oxford
Somogyi M (1952) Notes on sugar determination. J Biol Chem 195:19–23
Forciniti D (2000) Preparation of aqueous two-phase systems. In: Hatti-Kaul R (ed) Aqueous two-phase systems. Humana Press, Totowa
Cheetham PSJ (1979) Removal of Triton X-100 from aqueous solution using amberlite XAD-2. Anal Biochem 92:447–452
Tian M, Yan H, Row KH (2008) Extraction of glycyrrhizic acid and glabridin from licorice. Int J Mol Sci 9:571–577
Choi MPK, Chan KKC, Leung HW, Huie CW (2003) Pressurized liquid extraction of active ingredients (ginsenosides) from medicinal plants using non-ionic surfactant solutions. J Chromat A 983:153–162
Sun C, Xie Y, Tian Q, Liu H (2008) Analysis of glycyrrhizic acid and liquiritin in liquorice root with microwave-assisted micellar extraction and pre-concentration. Phytochem Anal 19:160–163
Sun C, Xie Y, Liu H (2007) Microwave-assisted micellar extraction and determination of glycyrrhizic acid and liquiritin in licorice root by HPLC. Chin J Chem Eng 15:474–477
Quina FH, Hinze WL (1999) Surfactant-mediated cloud point extractions: an environmentally benign alternative separation approach. Ind Eng Chem Res 38:4150–4168
Paleologos EK, Giokas DL, Karayannis MI (2005) Micelle-mediated separation and cloud-point extraction. Trends Anal Chem 24:426–436
Trescec A, Simic M, Branovic K, Gebauer B, Benko B (1999) Removal of detergent and solvent from solvent–detergent-treated immunoglobulins. J Chromat A 852:87–91
Calado V, Montgomery DC (2003) Planejamento de Experimentos usando o Statistica. E-papers Serviços Editoriais, Rio de Janeiro
Wang Y, Yan Y, Hu S, Han J, Xu X (2010) Phase diagrams of ammonium sulfate + ethanol/1-propanol/2-propanol + water aqueous two-phase systems at 298.15 K and correlation. J Chem Eng Data 55:876–881
Wang Y, Hu S, Han J, Yan Y (2010) Measurement and correlation of phase diagram data for several hydrophilic alcohol + citrate aqueous two-phase systems at 298.15 K. J Chem Eng Data 55:4574–4579
Jian HI, Liao XX, Zhu LW, Zhang WM, Jiang JX (2011) Synergism and foaming properties in binary mixtures of a biosurfactant derived from Camellia oleifera Abel and synthetic surfactants. J Colloid Interface Sci 359:487–492
Greve A, Kula MR (1991) Phase diagrams of new aqueous phase systems composed of aliphatic alcohols, salts and water. Fluid Ph Equilib 62:53–63
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ribeiro, B.D., Barreto, D.W. & Coelho, M.A.Z. Recovery of Saponins from Jua (Ziziphus joazeiro) by Micellar Extraction and Cloud Point Preconcentration. J Surfact Deterg 17, 553–561 (2014). https://doi.org/10.1007/s11743-013-1526-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-013-1526-5