Abstract
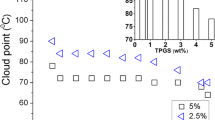
The effect of various organic additives, viz. sugars, ureas, alcohols, hydrotropes and bile salts on the clouding (phase separation) phenomenon of the amphiphilic antidepressant drug amitriptyline hydrochloride was investigated in the present study. All sugars lowered the cloud point (CP) due to their water structure-making property. Urea and alkylureas were found to lower the CP. In contrast, thioureas increased the CP slightly, but the presence of methyl group(s) had a similar effect in alkylureas. Short chain alcohols affected the CP insignificantly while higher ones decreased it, and medium chain alcohols showed peak behavior. Addition of hydrotropes and bile salts increased the CP at lower concentrations, while a decrease was observed at higher concentrations (like the medium chain alcohols). In addition, thermodynamic parameters were also evaluated but only for those additives which formed mixed micelles with the drug.






Similar content being viewed by others
References
Lang S (2002) Biological amphiphiles (microbial biosurfactants). Curr Opin Colloid Interface Sci 7:12–20
Tiddy GJT, Khan A (1999) Formulation science and technology-surfactants needed. Curr Opin Colloid Interface Sci 4:379–380
Schreier S, Malheiros SVP, de Paula E (2000) Surface active drugs: self-association and interaction with membranes and surfactants. Physicochemical and biological aspects. Biochim Biophys Acta 1508:210–234
Taboada P, Attwood D, Ruso JM, Garcia M, Sarmiento F, Mosquera V (1999) Influence of molecular structure on the ideality of mixing in micelles formed in binary mixtures of surface-active drugs. J Colloid Interface Sci 216:270–275
Attwood D, Tolley JA (1980) Self-association of analgesics in aqueous solution: association models for codeine, oxycodone, ethylmorphine and pethidine. J Pharm Pharmacol 32:761–765
Shaw DJ (1970) Introduction to colloid and surface chemistry, 2nd edn. Butterworths, London
Rosen MJ (2004) Surfactants and interfacial phenomena, 3rd edn. Wiley, New York
Raghupathy P, Mathiyarasu J, Joseph J, Phani KLN, Yegnaraman V (2005) Hydrotrope-driven disruption of micellar encapsulates for voltammetric detection of triclosan. J Electroanal Chem 584:210–214
Martin DW, Mayes PA, Rodwell VW (1981) Harper’s review of biochemistry, 18th edn. Maurzen Asia, Tokyo
Asano H, Ueno M (1997) Physicochemical properties of bile salts. In: Esumi K, Ueno M (eds) Structure-performance relationship in surfactants. Surfactant Science Series 112. Dekker, New York
Kabir-ud-Din, Rub MA, Naqvi AZ (2011) Aqueous amphiphilic drug (amitriptyline hydrochloride)–bile salt mixtures at different temperatures. Colloids Surf B 84:285–291
Hinze WL (1987) Organized surfactant assemblies in separation science. In: Hinze WL, Armstrong DW (eds) Ordered media in chemical separations, ACS symposium series 342, American Chemical Society, Washington, DC
Gu T, Galera-Gomez PA (1995) Clouding of Triton X-114: the effect of added electrolytes on the cloud point of Triton X-114 in the presence of ionic surfactants. Colloids Surf A 104:307–312
Al-Ghamdi AM, Nasr-El-Din HA (1997) Clouding of Triton X-114: the effect of added electrolytes on the cloud point of Triton X-114 in the presence of ionic surfactants. Colloids Surf A 125:5–18
Gu T, Galera-Gomez PA (1999) The effect of different alcohols and other polar organic additives on the cloud point of Triton X-100 in water. Colloids Surf A 147:365–370
Schott H (2001) Effect of inorganic additives on solutions of nonionic surfactants—XVI. Limiting cloud points of highly polyoxyethylated surfactants. Colloids Surf A 186:129–136
Bales BL, Zana R (2004) Cloud point of aqueous solutions of tetrabutylammonium dodecyl sulfate is a function of the concentration of counterions in the aqueous phase. Langmuir 20:1579–1581
Kumar S, Sharma D, Khan ZA, Aswal VK, Kabir-ud-Din (2006) Clouding phenomenon and SANS studies on tetra-n-butylammonium dodecylsulfate micellar solutions in the absence and presence of salts. J Colloid Interface Sci 302:315–321
Karlstrom G (1985) A new model for upper and lower critical solution temperatures in poly(ethylene oxide) solutions. J Phys Chem 89:4962–4964
Tasaki K (1996) Poly(oxyethylene)–water interactions: a molecular dynamics study. J Am Chem Soc 118:8459–8469
Kumar S, Sharma D, Kabir-ud-Din (2000) Cloud point phenomenon in anionic surfactant + quaternary bromide systems and its variation with additives. Langmuir 16:6821–6824
Kumar S, Sharma D, Khan ZA, Kabir-ud-Din (2002) Salt-induced cloud point in anionic surfactant solutions: role of the headgroup and additives. Langmuir 18:4205–4209
Kumar S, Sharma D, Kabir-ud-Din (2003) Temperature-[salt] compensation for clouding in ionic micellar systems containing sodium dodecyl sulfate and symmetrical quaternary bromides. Langmuir 19:3539–3541
Kim EJ, Shah DO (2002) Cloud point phenomenon in amphiphilic drug solutions. Langmuir 18:10105–10108
Alam MS, Naqvi AZ, Kabir-ud-Din (2007) Influence of organic additives on the clouding phenomena of promethazine hydrochloride solutions. Colloid Polym Sci 285:1573–1579
Alam MS, Naqvi AZ, Kabir-ud-Din (2007) Influence of electrolytes/non-electrolytes on the cloud point phenomenon of the aqueous promethazine hydrochloride drug solution. J Colloid Interface Sci 306:161–165
Alam MS, Naqvi AZ, Kabir-ud-Din (2007) Role of surfactants in clouding phenomenon of imipramine hydrochloride. Colloids Surf B 57:204–208
Alam MS, Kabir-ud-Din, Mandal AB (2010) Thermodynamics at the cloud point of phenothiazine drug chlorpromazine hydrochloride-additive systems. J Chem Eng Data 55:1693–1699
Britton HTS (1942) Hydrogen ions; their determination and importance in pure and industrial chemistry, vol 2, 3rd edn. Chapman, London
Attwood D, Florence AT (1983) Surfactant systems: their chemistry, pharmacy and biology. Chapman and Hall, New York
Alam MS, Kabir-ud-Din, Mandal AB (2010) Thermodynamics of the amphiphilic drug amitriptyline hydrochloride-surfactant/polymer systems at the cloud point. J Dispers Sci Technol 31:1721–1726
Kabir-ud-Din, Rub MA, Naqvi AZ (2010) Mixed micelle formation between amphiphilic drug amitriptyline hydrochloride and surfactants (conventional and Gemini) at 293.15–308.15 K. J Phys Chem B 114:6354–6364
Kabir-ud-Din, Rub MA, Naqvi AZ (2011) Mixed micelles of amphiphilic drug promethazine hydrochloride and surfactants (conventional and Gemini) at 293.15 K to 308.15 K: composition, interaction and stability of the aggregates. J Colloid Interface Sci 354:700–708
Katzung BG (2004) Basic and clinical pharmacology, 9th edn. McGraw Hill, New York
Lakshmi TS, Nandi PK (1976) Effects of sugar solutions on the activity coefficients of aromatic amino acids and their N-acetyl ethyl esters. J Phys Chem 80:249–252
Nozaki Y, Tanford C (1963) The solubility of amino acids and related compounds in aqueous urea solutions. J Biol Chem 238:4074–4081
Enea O, Jolicoeur CJ (1982) Heat capacities and volumes of several oligopeptides in urea-water mixtures at 25 °C. Some implications for protein unfolding. J Phys Chem 86:3870–3881
Briganti G, Puvvada S, Blankschtien D (1991) Effect of urea on micellar properties of aqueous solutions of nonionic surfactants. J Phys Chem 95:8989–8995
Candau S, Zana R (1981) Effect of alcohols on the properties of micellar systems: III. Elastic and quasielastic light scattering study. J Colloid Interface Sci 84:206–219
Forland GM, Samseth J, Hoiland H, Mortensen K (1994) The effect of medium chain length alcohols on the micellar properties of sodium dodecyl sulfate in sodium chloride solutions. J Colloid Interface Sci 164:163–167
Caponetti E, Chillura Martino D, Floriano MA, Triolo R (1997) Localization of n-alcohols and structural effects in aqueous solutions of sodium dodecyl sulfate. Langmuir 13:3277–3283
Almgren M, Swarup S, Lofroth JE (1985) Effect of formamide and other organic polar solvents on the micelle formation of sodium dodecyl sulfate. J Phys Chem 89:4621–4626
De Gennes PG, Taupin C (1982) Microemulsions and the flexibility of oil/water interfaces. J Phys Chem 86:2294–2304
Abu-Hamdiyyah M, Rahman IA (1985) Strengthening of hydrophobic bonding and the increase in the degree of micellar ionization by amphiphiles and the micelle-water distribution coefficient as a function of the surfactant chain length in sodium alkyl sulfates. J Phys Chem 89:2377–2384
Reekmans S, Luo H, Van der Auweraer M, De Schryver FC (1990) Influence of alcohols and alkanes on the aggregation behavior of ionic surfactants in water. Langmuir 6:628–637
Mitchell DJ, Ninham BW (1981) Micelles, vesicles and microemulsions. J Chem Soc, Faraday Trans 2(77):601–629
Rao URK, Manohar C, Valaulikar BS, Iyer RM (1987) Micellar chain model for the origin of the viscoelasticity in dilute surfactant solutions. J Phys Chem 91:3286–3291
Majhi PR, Mukerjee K, Moulik SP, Sen S, Sahu NP (1999) Solution properties of a saponin (Acaciaside) in the presence of Triton X-100 and Igepal. Langmuir 15:6624–6630
Ghosh S, Moulik SP (1999) The clouding behaviours of binary mixtures of polyoxyethylene (10) Cetylether (Brij-56) with polyvinyl alcohol (PVA) and methyl cellulose (MC). Indian J Chem A 38:201–208
Blankschtein D, Thurston GM, Benedek GB (1986) Phenomenological theory of equilibrium thermodynamic properties and phase separation of micellar solutions. J Chem Phys 85:7268–7288
Liu CL, Nikas YJ, Blankschtein D (1996) Novel bioseparations using two-phase aqueous micellar systems. Biotechnol Bioeng 52:185–192
Acknowledgments
The authors are thankful to the Council of Scientific and Industrial Research (CSIR), New Delhi, India for a research grant (No. 01(2208)/08/EMR–II).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Rub, M.A., Asiri, A.M., Kumar, D. et al. Effect of Organic Additives on the Phase Separation Phenomenon of Amphiphilic Drug Solutions. J Surfact Deterg 15, 765–775 (2012). https://doi.org/10.1007/s11743-012-1354-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-012-1354-z