Abstract
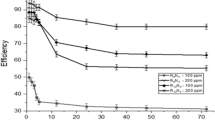
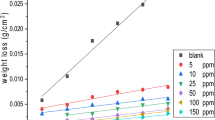
Seven cationic surfactants: 1-methyl-3-tetradecyl imidazolium bromide, 1-methyl-3-hexadecyl imidazolium bromide, N,N-tetradecyl pyridinium bromide, N,N-hexadecyl pyridinium bromide, N,N-dimethyl-N-ethylbenzyl ammonium bromide, N,N-dimethyl-N-ethylbenzyl ammonium laurate and N,N-dimethyl-N-ethylbenzyl ammonium acetate, were investigated at different doses (10, 25, 50, 100, and 200 ppm) as corrosion inhibitors for steel grade API 5L X52 in hydrochloric acid 2 M using a weight loss technique, impedance and polarization resistance methods. The corrosion inhibition of steel grade API 5L X52 of the cationic surfactants was attributed to their molecular structure (heterocyclic ring, hydrophobic chain length and counterion) that enhances adsorption onto steel surface. The best protective efficiency of the film was higher than 90% (N,N-Dimethyl-N-ethylbenzyl ammonium acetate). It is important to know how organic inhibitor films grown on the metallic surface in order to achieve superior corrosion inhibition, hence experimental findings were described by Langmuir adsorption isotherm. The Electrochemical Impedance Spectroscopy spectrums were fitted by means of the Voigt model.








Similar content being viewed by others
Abbreviations
- MTIm:
-
1-Methyl-3-tetradecyl imidazolium bromide
- MHIm:
-
1-Methyl-3-hexadecyl imidazolium bromide
- Tpy:
-
N,N-Tetradecyl pyridinium bromide
- Hpy:
-
N,N-Hexadecyl pyridinium bromide
- DEBABr:
-
N,N-Dimethyl-N-ethylbenzyl ammonium bromide
- DEBAL:
-
N,N-Dimethyl-N-ethylbenzyl ammonium laurate
- DEBAA:
-
N,N-Dimethyl-N-ethylbenzyl ammonium acetate
References
Negm NA, Mohamed AS (2004) Surface and thermodynamics properties of diquaternary bola-form amphiphiles containing and aromatic spacer. J Surfactant Deterg 7:23–30
Martin RL (2003) Corrosion inhibitors for oil and gas production. In: Cramer SD, Covino BS (eds) ASM handbook. ASM International, New York, vol 13A, pp 878–886
Kane RD (2003) Corrosion in petroleum production operations. In: Cramer SD, Covino BS (eds) ASM handbook. ASM International, New York, vol 13C, pp 922–966
Negm NA, Aiad IAJ (2007) Synthesis and characterization of multifunctional surfactants in oil-field protection applications. J Surfactant Deterg 10:87–92
Bhaskaran R, Palaniswamy N, Rengaswamy NS, Jayachandran M (2003) Global cost of corrosion-a historical review. In: Cramer SD, Covino BS (eds) ASM handbook. ASM International, New York, vol 13B, pp 621–628
Wojtanowicz AK (2008) Environmental control technology for oilfield processes. In: Orszulik ST (ed) Environmental technology in the oil industry. Springer, Berlin, pp 17–51
Buck E (1995) Inhibitors. In: Babonian R (ed) Corrosion test y standards: application and interpretation. ASTM, Fredericksburg, pp 403–410
Pérez-Navarrete JB, Likhanova NV, Plomar ME, Herrea H, Romer M (2008) Electrochemical evaluation of new synthesised ionic liquids as steel corrosion inhibitors in H2SO4 1.0 M. XXI Week of Chemistry and Chemical Education, Mexico City, pp 581–592
Negm NA, Morsy SMI (2005) Corrosion inhibition of triethanolammonium bromide mono and dibenzoate as cationic inhibitors in an acidic medium. J Surfactant Deterg 8:283–287
Morelli JJ, Szajer G (2001) Analysis of surfactants: part II. J. Surfactant Deterg 4:75–83
Bellmann C (2008) Surface modification by adsorption of polymers and surfactants. In: Stamm M (ed) Polymer surfaces and interfaces. Springer, Berlin, pp 235–259
Abd El–Maksoud SA (2008) The effect of organic compounds on the electrochemical behaviour of steel in acidic media. A review. Int J Electrochem Sci 3:528–555
Pérez-Navarrete JB (2009) Síntesis y evaluación de surfactantes catiónicos (Spanish). UAM, Mexico
Selvi T, Raman V, Rajendran N (2003) Corrosion inhibition of mild steel by benzotriazole derivatives in acidic medium. J Appl Electrochem 33:1175–1182
Syed Azim S, Muralidharan S, Venkatakrishna Iyer S (1995) Studies on the influence of iodide ions on the synergistic inhibition of the corrosion of mild steel in an acidic solution. J Appl Electrochem 25:495–500
Wagner C, Traud W (2006) On the interpretation of corrosion process trough the superposition of electrochemical partial processes and on the potential of mixed electrodes. Corrosion 62:844–855
Tang YM, Chen Y, Yang WZ, Liu Y, Yin XS, Wang JT (2008) Electrochemical and theoretical studies of thienyl-substituted amino triazoles on corrosion inhibition of copper in 0.5 M H2SO4. J Appl Electrochem 38:1553–1559
Stern M, Geary AL (1957) Electrochemical polarization, I. A theoretical analysis of the shape of polarization curves. J Electrochem Soc 104:56–93
Li WH, He Q, Zhang ST, Pei CL, Hou BR (2008) Some new triazole derivatives as inhibitors for mild steel corrosion in acidic medium. J Appl Electrochem 3:289–295
Migahed MA, Abd-El-Raouf M, Al-Sabagh AM, Abd-El-Bary HM (2006) Corrosion inhibition of carbon steel in acid chloride solution using ethoxylated fatty alkyl amine surfactants. J Appl Electrochem 36:395–402
Macdonald DD (2007) Theory of passive film stability. ECS Trans 2:73–81
Chin RJ, Nobe K (1972) Electrodissolution kinetics of iron in chloride solutions, III. Acidic solutions. J Electrochem Soc 119:1457–1461
Deyab MA (2007) Effect of cationic surfactant and inorganic anions on the electrochemical behaviour of carbon steel in formation water. Corros Sci 49:2315–2328
Epelboin I, Keddam M, Takenouti H (1972) Use of impedance measurements for the determination of the instant rate of metal corrosion. J Appl Electrochem 2:71–79
Ruthven DM (1984) Principles of adsorption and adsorption process. Wiley, USA
Yashkin SN, Schuster RH (2003) Investigation of energetic heterogeneity of carbon black surfaces during adsorption of chromatographically low concentrations of n-pentane. Russ Chem Bull Int Ed 52:2360–2368
Pérez-Navarrete JB, Olivares-Xometl CO, Likhanova NV (2010) Adsorption and corrosion inhibition of amphiphilic compounds on steel pipeline grade API 5L X52 in sulphuric acid 1 M. J Appl Electrochem 40:1605–1617
Yang CH (1993) Statistical mechanical aspects of adsorption systems obeying the Temkin isotherm. J Phys Chem 97:7097–7101
Parsons R (1959) The relation between the capacity of the electrode double layer and the adsorption of surface active material. Trans Faraday Soc 55:999–1006
Kerner Z, Pajkossy T (2000) On the origin of capacitance dispersion of rough electrodes. Electrochim Acta 46:207–211
Tvardovski A, Tondeur D, Favre E (2003) Description of multicomponent adsorption and absorption phenomena from a single viewpoint. J Coll Interf Sci 265:239–244
Le Mehaute A, Crepy G (1983) Introduction to transfer and motion in fractal media: the geometry of kinetics. Solid State Ion 9:17–30
Macdonald DD (2006) Reflections on the history of electrochemical impedance spectroscopy. Electrochim Acta 51:1376–1388
Orazem ME, Agarwal P, Deslouis C, Tribollet B (1996) Application of measurement models to electrohydrodynamic impedance spectroscopy. J Electrochem Soc 143:948–960
Kanoun O, Tröltzsch U, Tränkler HR (2006) Benefits of evolutionary strategy in modelling of impedance spectra. Electrochim Acta 51:1453–1461
Urquidi-Macdonald M, Real S, Macdonald DD (1990) Applications of Kramers-Kronig transformation in the analysis of electrochemical impedance data-III, stability and linearity. Electrochim Acta 35:1559–1566
Esteban JM, Orazem ME (1991) On the application of the Kramers-Kronig relations to evaluate the consistency of electrochemical impedance data. J Electrochem Soc 138:67–76
Agarwal P, Orazem ME, García-Rubio LH (1993) Application of the Kramers-Kronig relations in electrochemical impedance spectroscopy. In: Scully JR, Silverman DC, Kendig MW (eds) Electrochemical impedance: analysis and interpretation. ASTM, Fredericksburg, pp 115–137
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Palomar, M.E., Olivares-Xometl, C.O., Likhanova, N.V. et al. Imidazolium, Pyridinium and Dimethyl-Ethylbenzyl Ammonium Derived Compounds as Mixed Corrosion Inhibitors in Acidic Medium. J Surfact Deterg 14, 211–220 (2011). https://doi.org/10.1007/s11743-010-1236-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-010-1236-1