Abstract
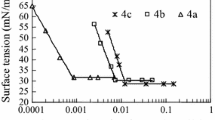
A homologous series of surface active 2-(alkanoylamino)ethyldimethylamine-N-oxides, EDA-p(O), and 3-(alkanoylamino)propyldimethylamine-N-oxides, PDA-p(O), were synthesized. Their aqueous stock solutions were processed by the automatically operating apparatus to remove surface-active contamination and provide chemical purity at the air/water interface. In case of 3-(tetradecanoylamino)propyldimethylamine-N-oxide, PDA-14(O), the difference between equilibrium surface tension values of the purified surfactant solutions and the corresponding values of the solutions prepared from the “as received” compounds amounts to 15 mN m−1. Moreover, in presence of the surface-active contaminants time needed to reach equilibrium surface tension values is over 2 h. For the solution of the “surface-chemically pure” grade the change of the surface tension within adsorption time is negligible and the equilibrium is reached instantaneously. Physicochemical properties of obtained surface-chemically pure aqueous solutions of N-oxides of alkylamidoamines and adsorption parameters (standard free energy of adsorption, ΔG°ads, surface excess by saturation surface concentration, Γ∞, minimum surface area demand per molecule adsorbed, A min) were evaluated from the equilibrium surface tension versus concentration isotherms at the air/water interface using Gibbs’s equation. The introduction of the CH2 moiety into the intermediate part of molecule causes a slight decrease of the hydrophobic character of surfactant. Also the minimum surface area demand, A min, is slightly greater for PDA series than for the corresponding EDA derivatives. Surface potential measurements were performed in addition to surface tension studies. Electric surface potential versus concentration isotherms was determined. Surface potential increases with increasing surfactant’s bulk concentration for all investigated compounds. At highest concentrations, where interface is almost saturated, changes of surface potential become almost negligible.








Similar content being viewed by others
References
Sauer JD (1990) Amine oxides. In: Richmond JM (ed) Cationic surfactants: organic chemistry. Surfactant science series, vol 34. Marcel Dekker, Inc., USA, pp 275–295
Vaikunth SP (1999) Synthesis of tertiary amine oxides. US Patent 5,866,718
Sprung JA (1962) Polivinyl alcohol photographic silver halide emulsions. Ger Patent 1,139,738
Cook EW, Moss PH (1944) Quaternary ammonium compounds. US Patent 2,459,062
Dassanayake NL, Schlitzer RL, Park J (1997) Use of amidoamines in ophthalmic compositions. US Patent 5,631,005
Proietto V, Salhi A (1991) N-Substituted lauramides, their preparation and compositions in which they are present. US Patent 5,068,064
Procter & Gamble Company (1965) Detergent compositions. Brit Patent 998,685
Kubo M, Terasaki H, Sakai T, Fujio A (1999) Production of amine oxide. Jap Patent 11005775
Morita H, Chiba Y, (1999) Production of amidamine oxide compound excellent in stability and surface composition derived from the same compound. Jap Patent 11152260.
Prodo KW, Bender RW (1978) Surface-active agent. US Patent 4,077,990
Schrader K–H (1990) Hair dyeing and tinting product. Eur Patent Appl 0,367,926
Pantke H, Vogel K (1976) Agents for improving wet fastness properties. US Patent 3,995,996
D’Ambrosio R, Jakubicki G, Arvanitidou E, Gambogi J (1999) High foaming grease cutting light duty liquid composition containing a C10 alkyl amido propyl dimethyl amine oxide. US Patent 5,998,347
Flanagan JJ (1980) Surfactant system. US Patent 4,203,872
Mori N, Takano K (1998) Detergent composition for hard surface. Jap Patent 10176187
Bates PJM, Fredj A, Housmekerides ChE, Jones RJ (1997) Low sudsing liquid detergent composition. Eur Patent Appl 0,798,370
Anonymous (2000) Final report on the safety assessment of cocamidopropylamine oxide. Int J Toxicol 19(Suppl. 2):1
Michaels EB (1979) Antimicrobial composition and method for using the same. US Patent 4,145,436
Lynch DM, Lover MJ Singer AJ, Rhodes III WE (1979) Alkyl amine oxide toxicants. US Patent 4,179,504
Stepan Studies: 99-011C and 02-016A (2003) http://www.stepan.com
Mysels KJ, Florence AT (1973) The effect of impurities on dynamic surface tension-basis for a valid surface purity criterion. J Colloid Interface Sci 43:577
Lunkenheimer K, Miller R (1979) On the purity of aqueous surfactant solutions and the dynamic surface tension behaviour. Tenside Deterg 16:312
Lunkenheimer K, Pergande HJ, Krüger L (1987) Apparatus for programmed high-performance purification of surfactant solutions. Rev Sci Instrum 58:2313
Lunkenheimer K, Miller RJ (1987) A criterion for judging the purity of adsorbed surfactant layers. J Colloid Interface Sci 120:176
Lunkenheimer K, Wantke KD (1981) Determination of the surface tension of surfactant solutions applying the method of Lecomte du Noüy (ring tensiometer). Colloid Polymer Sci 259:354
Potter EF (1937) Monomolecular films of α-aminostearic acid, stearic acid, and heptadecylamine. J Am Chem Soc 59:1883
Lunkenheimer K, Barzyk W, Hirte W, Rudert R (2003) Adsorption properties of soluble, surface-chemically pure n-alkanoic acids at the air/water interface and the relationship to insoluble monolayer and crystal structure properties. Langmuir 19:6140
Beyer K, Klingenberg M (1978) Interaction of an amine oxide detergent with lecitin vesicles as studied by nuclear magnetic resonance. Biochemistry 17:1424
Adamson AW (1993) Physical chemistry of surfaces. Wiley & Sons Ltd, New York
Piłakowska-Pietras D, Lunkenheimer K, Piasecki A (2004) Adsorption behavior of surface-chemically pure N-alkyl-N-(2-hydroxyethyl)aldonamides at the air/water interface. Langmuir 20:1572
Lunkenheimer K, Haage K, Hirte W (1999) Novel results on the adsorption properties of n-alkyldimethylphosphine oxides at the air/water interface. Langmuir 15:1052
Lunkenheimer K, Burczyk B, Piasecki A, Hirte R (1991) Effects of stereochemistry and alternation on the adsorption properties of surface-active cis- and trans-2-n-alkyl-5-hydroxy-1, 3-dioxanes. Langmuir 7:1765
Acknowledgments
Support for this work by the Polish State Committee for Scientific Research, Grant No. 3-TO9B 075 27 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Piasecki, A., Piłakowska-Pietras, D., Baran, A. et al. Synthesis and Properties of Surface Chemically Pure Alkylamidoamine-N-oxides at the Air/Water Interface. J Surfact Deterg 11, 187–194 (2008). https://doi.org/10.1007/s11743-008-1070-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-008-1070-x