Abstract
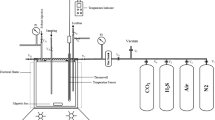
Modern industrial sulfonation of linear alkylbenzene (LAB) in falling film reactors produces p-alkylbenzene sulfonic acid (HLAS), which is usually neutralized with caustic soda (NaOH) and used in detergent formulations. During the sulfonation process, other products such as anhydrides and sulfones are also formed. Four reactions are proposed to occur during aging and hydrolysis. As a consequences, approximately 25% of sulfones and 75% of unreacted LAB are removed during aging and are transformed into additional active matter. Anhydrides are completely eliminated during hydrolysis. On the other hand, sulfones formed during the process are difficult to remove once formed. The goal of this work was to optimize the various operating conditions of the sulfonation process and to understand the various secondary reactions that occur therein so as to obtain a maximal active ingredient concentration in the final sulfonated product and a minimum of unsulfonated matter. Formation of sulfones can be minimized by using an SO3/LAB molar ratio slightly lower than the theoretical optimum.
Similar content being viewed by others
Abbreviations
- FFR:
-
falling film reactors
- FO:
-
free oil
- HLAS:
-
p-alkylbenzene sulfonic acid
- HPLC:
-
high-performance liquid chromatography
- LAB:
-
linear alkylbenzene
References
Alamany, M., A. Mompeon, and A. Moreno, Influencia de la sulfonación con SO3 sobre las propiedades físicas de los sulfonatos sódicos de alquilbenceno lineal, Proceedings of IV Jornadas del Comité Español de la Detergencia (CED), Barcelona, Spain, 1973.
Moreno, A., J. Bravo, and J.L. Berna, Influence of Unsulfonated Material and Its Sulfone Content on the Physical Properties of Linear Alkylbenzene Sulfonates, J. Am. Oil Chem. Soc. 65:6 (1988).
de Groot, W.H., Sulfonation Technology in the Detergent Industry, Kluwer Academic Publishers, Norwell, MA, 1991.
Cohen, L., A. Moreno, and J.L. Berna, Analysis and identification of Minor Products in Linear Alkylbenzene Sulfonation, Tenside Surfactatts Deterg. 33:6 (1996).
Gilbert E.E., Sulfonation and Related Reactions, Interscience Publishers, New York, 1965.
Roberts, D.W., and J.O. Morley, A Molecular Orbital Study of the Mechanism of Alkylbenzene Sulfonation, Proceedings of XXVII Jornadas del Comité Espanol de la Detergencia (CED), Barcelona, Spain, 1997, p. 145.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Moreno, A., Bengoechea, C., Bravo, J. et al. A contribution to understanding secondary reactions in linear alkylbenzene sulfonation. J Surfact Deterg 6, 137–142 (2003). https://doi.org/10.1007/s11743-003-0257-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11743-003-0257-2