Abstract
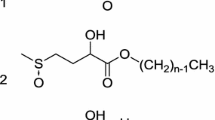
Dehydroabietates with poly(ethylene oxide) chains of average m=12, 17, and 45 units [DeHab(E) m ] were synthesized. The adsorption at the liquid-vapor interface was measured, and the adsorbed amount and critical micelle concentrations (CMC) were determined. The foamability, the foam stability, wetting properties, and cloud points, with and without salt content, were studied. The results were compared with common linear alkyl ethoxylates, nonylphenol ethoxylates, and cholesterol ethoxylates. The dehydroabietic acid as hydrophobe was found to result in the same CMC as a linear dodecyl chain. DeHab(E)45 was found to be insoluble above 400 mg/L, but the surface tensions at lower concentrations were similar to those of the C11–13E38–40 surfactants, which exhibit CMC in aqueous media. The foaming behavior of the DeHab(E)12 and DeHab(E)17 surfactants was about the same as for common linear C n E m surfactants. The foamability as well as the foam stability increased with ethylene oxide (EO) chain length. The cloud point was depressed by increased salt concentration and increased with the number of EO units in the head group. The cloud point was significantly lower than for the corresponding surfactant with a dodecyl chain with similar EO chain length. The wetting results, obtained by measuring the contact angle at similar surface tensions, indicate that surfactants of the DeHab(E) m type are more efficient wetting agents than both disaccharide sugar surfactants and C n E m type surfactants.
Similar content being viewed by others
Abbreviations
- CMC:
-
critical micelle concentration
- DeHab(E) m :
-
dehydroabietates with poly(ethylene oxide) chains of m=12, 17, or 45 units
- EO:
-
ethylene oxide
- IR:
-
infrared
- MS:
-
mass spectrometry
- NMR:
-
nuclear magnetic resonance
- NP-EO m (or NP-EO n ):
-
nonylphenolethoxylate of m (or n) number of EO units
- PEG:
-
polyethylene glycol
References
Rico-Lattes, I., and A. Lattes, Synthesis of New Sugar-Based Surfactants Having Biological Applications: Key Role of Their Self-Association, Colloids Surf. A 123–124:37 (1997).
Söderberg, I., C.J. Drummond, D.N. Furlong, S. Godkin, and B. Matthews, Non-ionic Sugar-Based Surfactants: Self-Assembly and Air/Water Interfacial Activity, Colloids Surf. A 102:91 (1995).
Aveyard, R., B.P. Binks, J. Chen, J. Esquena, P.D.I. Fletcher, B.R. Buscall, and S. Davies, Surface and Colloid Chemistry of Systems Containing Pure Sugar Surfactants Langmuir 14:4699 (1998).
Retailleau, L., A. Laplace, H. Fensterbank, and C. Larpent, Synthesis, Structural Analysis, and Properties of N-Alkylglucosyl(meth)acrylamides: New Reactive Sugar Surfactants, J. Org. Chem. 63:608 (1998).
Garofalakis, G., B.S. Murray, and D.B. Sarnay, Surface Activity and Critical Aggregation Concentration of Pure Sugar Esters with Different Sugar Headgroups, J. Colloid Interface Sci. 229:391 (2000).
Sarney, D.B., H. Kapeller, G. Fregapane, and E.N. Vulfson, Chemo-enzymatic Synthesis of Disaccharide Fatty Acid Esters, J. Am. Oil Chem. Soc. 71:711 (1994).
Balzer, D., Cloud Point Phenomena in the Phase Behavior of Alkyl Polyglucosides in Water, Langmuir 9:3375 (1993).
Boyd, B.J., C.J. Drummond, I. Krodkiewska, and F. Grieser, How Chain Length, Headgroup Polymerization, and Anomeric Configuration Govern the Thermotropic and Lyotropic Liquid Crystalline Phase Behavior and the Air-Water Interfacial Adsorption of Glucose-Based Surfactants, Langmuir. 16:7359 (2000).
Zhang, T., and R.E. Marchant, Novel Polysaccharide Surfactants: The Effect of Hydrophobic and Hydrophilic Chain Length on Surface Active Properties, J. Colloid Interface Sci. 177:419 (1996).
Kjellin, U.R.M., P.M. Claesson, and E.N. Vulfson, Studies of N-Dodecyllactobionamide, Maltose 6′-O-Dodecanoate, and Octyl-β-glucoside with Surface Tension, Surface Force, and Wetting Techniques, Langmuir 17:1941 (2001).
Folmer, B.M., M. Svensson, K. Holmberg, and W. Brown, The Physicochemical Behavior of Phytosterol Ethoxylates, J. Colloid Interface Sci. 213:112 (1999).
Folmer, B.M., K. Holmberg, E. Gottberg-Klingskog, and K. Bergström, Fatty Amide Ethoxylates: Synthesis and Self-Assembly, J. Surfact. Deterg. 4:175 (2001).
Khachadurian, A., C.H. Fung, T. van Es, and F.F. Davis, Polyoxyethylated Cholesterol Derivatives—Organic Synthesis, Cellular Uptake, and Effect on Lipid Metabolism in Cultured Skin Fibroblasts, Biochim. Biophys. Acta 665:434 (1981).
Mueller-Goymann, C., Liquid Crystals in Emulsions, Creams, and Gels Containing Ethoxylated Sterols as Surfactant, Pharm. Res. 4:154 (1984).
Shibamoto, T., M. Fukushima, and T. Suzuki, Cosmetic, Japanese Patent JP-62,187,404, Pola Chemical Industries Inc. (1987).
Barla, P., K. Larsson, H. Ljusberg-Wahren, T. Norin, and K. Roberts, Phase Equilibria in a Ternary System Saponin-Sunflower Oil Monoglycerides-Water: Interactions Between Aliphatic and Alicyclic Amphiphiles, J. Sci. Food Agric. 30:864 (1979).
Persson, M., P. Stenius, G. Ström, L. Ödberg, I. Bolmgren, H. Ljusberg-Wahren, and T. Norin, Solubilization, Micellization, and Phase Equilibria of Polyoxyethylene Derivatives of Dehydroabietic Acid, J. Phys. Chem. 84:1557 (1980).
Piispanen, P.S., S. Byström, M. Svensson, B. Kronberg, I. Blute, and T. Norin, Synthesis and Characterization of Surface-Active Compounds Derviced from Cholesterol Derivatives and Glucose, J. Surfact. Deterg. 345:351 (2002).
Goossens, A., M.H. Beck, E. Haneke, J.P. McFadden, S. Nolting, G. Durupt, and G. Ries, Adverse Cutaneous Reactions to Cosmetic Allergens, Contact Dermatitis 40:112 (1999).
Amorosa, L.F., C.P. Martucci, N.R. Stevenson, and A.K. Khachadurian, The Effects of Polyethylated Cholesterol on Fecal Bile Acids and Nitrogen and on Cholesterol Balance in Rats, Lipids 26:209 (1991).
Hamunen, A., and A. Hirschfeldt, Sterols from Unsaponifiable Constituents of Tall Oil—A Wood-Based Raw Material for the Cosmetic, Pharmaceutical, and Food Industries, Seifen, Oele, Fette, Wachse 112:261 (1986).
Kaluza, U., and K. Taeger, Struktur und Eigenschaften von Alkanol-Ethoxylaten, Tenside Surfact. Deterg. 33:46 (1996).
Matsumura, S., K. Imai, S. Yoshikawa, K. Kawada, and T. Uchibori, Surface Activities, Biodegradability and Antimicrobial Properties of n-Alkyl Glucosides, Mannosides and Galactosides, J. Am. Oil Chem. Soc. 67:996 (1990).
Matsumura, S., Y. Kawamura, S. Yoshikawa, K. Kawada, and T. Uchibori, Surface Activities, Biodegradability and Antimicrobial Properties of Glucosamine Derivatives Containing Alkyl Chains, J. Am. Oil Chem. Soc. 70:17 (1993).
Hedman, B.E.O., Tall Oil Products as Raw Materials for Surfactant Synthesis, Licensiate Thesis, Royal Institute of Technology (KTH), Stockholm, Sweden, 2000, p. 25.
Akatsuka, T., O. Kodama, K. Matsuo, and Y. Esaki, Agent to Control Rice Blast, Japanese Patent JP-63,017,806, Arakawa Chem. Ind. Co. Ltd. (1986).
Akatsuka, T., O. Kodama, K. Matsuo, and Y. Esaki, Agent to Control Rice Blast, Japanese Patent JP-19,880,125 Arkawa Chem. Ind. Co. Ltd. (1986).
American Standard Test Methods, a Standard Test Method for Foaming Properties of Surface-Active Agents, D1173-53 (2001).
Ross, J., and G.D. Miles, An Apparatus for Comparison of Foaming Properties of Soaps and Detergents, Oil Soap 18:99 (1941).
Ameri, M., D. Attwood, J. Collett, and C. Booth, Self-Assembly of Alcohol Ethoxylate Non-ionic Surfactants in Aqueous Solution, J. Chem. Soc., Faraday Trans. 93:2545 (1997).
Söderlund, H., J. Sjöblom, T. Wärnheim, Phase Diagrams and Solution Properties of Ethoxylated Cholesterols, J. Dispersion Sci. Technol. 10:131 (1989).
Svensson, M., Surfactants Based on Sterols and Other Alicyclic Compounds, in Novel Surfactants, edited by K. Holmberg, Marcel Dekker, New York, 1998, p. 179.
Schick, M. (ed.), Nonionic Surfactants, Marcel Dekker, New York, 1966.
Cox, M.F., Effect of Alkyl Carbon Chain Length and Ethylene Oxide Content on the Performance of Linear Alcohol Ethoxylates, J. Am. Oil Chem. Soc. 66:367 (1989).
Florin, E., R. Kjellander, and J.C. Eriksson, Salt Effects on the Cloud Point of the Poly(ethylene oxide) + Water System, J. Chem. Soc. Faraday Trans. 80:2889 (1984).
Mitchell, D.J., G.J.T. Tiddy, L. Waring, T. Bostock, and M.P. McDonald, Phase Behaviour of Polyoxyethylene Surfactants with Water, J. Chem. Soc. Faraday Trans. 179:975 (1983).
Garrett, P.R., and P.L. Gratton, Dynamic Surface Tensions, Foam and the Transition from Micellar Solution to Lamellar Phase Dispersion, Colloids Surf. A 103:127 (1995).
Drummond, C.J., and D. Wells, Nonionic Lactose and Lactitol-Based Surfactants: Comparison of Some Physico-chemical Properties, Colloids Surf. A 141:131 (1998).
Gau, C.-S., and G. Zografi, Relationships Between Adsorption and Wetting of Surfactant Solutions, J. Colloid Interface Sci. 140:1 (1990).
Bargeman, D., and F. Van Voorst Vader, Effect of Surfactants on Contact Angles at Nonpolar Solids, J. Colloid Interface Sci. 42:467 (1972).
Kjellin, U.R.M., P.M. Claesson, and P. Linse, Surface Properties of Tetra(ethylene oxide) dodecyl Amide Compared with Poly(ethylene oxide) Surfactants. 1. Effect of the Headgroup on Adsorption, Langmuir 18:6745 (2002).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Piispanen, P.S., Kjellin, U.R.M., Hedman, B. et al. Synthesis and surface measurements of surfactants derived from dehydroabietic acid. J Surfact Deterg 6, 125–130 (2003). https://doi.org/10.1007/s11743-003-0255-4
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11743-003-0255-4