Abstract
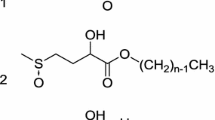
Maltose long-chain fatty acid esters (MFAE), esterified at the 6 and 6′ position, were synthesized with stearic, palmitic, myristic, and oleic groups. Synthesis yields were 15–20% based on initial maltose present, and structural confirmation was obtained using plasma desorption mass spectrometry and nuclear magnetic resonance spectroscopy. These surfactants have surface tensions in the range of 34–36 dyn/cm at their critical micelle concentrations (CMC) of approximately 10−5–10−6 mol/L. The increased chain lengths have a marked effect, reducing CMC values for MFAE by approximately three orders of magnitude over similar carbohydrate-based dodecyl chain sources. Within chain lengths between 14 and 18 carbons, the rate of change in CMC is significant and decreases with increasing chain length for MFAE. The melting points of MFAE are approximately 40°C, and the heat capacities range from 1.6 to 1.9 J/g·K. These numbers are comparable to those of sucrose esters, indicating their applicability in similar uses. However, because MFAE, unlike sucrose, possess an anomeric carbohydrate carbon position, these surfactants maintain their reducing nature and are susceptible to further derivatization. They are also synthesized from renewable, economical carbohydrates and lipids and may provide an excellent alternative to pertrochemical-derived products.
Similar content being viewed by others
References
Whistler, R.L., and J.N. BeMiller, Monosaccharides, in Carbohydrate Chemistry for Food Scientists, edited by R.L. Whistler and J.N. BeMiller, Eagan Press, St. Paul, 1997, p. 1.
Egan P.A., Surfactants from Biomass, CHEMTECH (December): 758 (1989).
Hass, H.B., Early History of Sucrose Esters, in Sugar Esters, edited by H.B. Hass and The Sugar Research Foundation, Noyes Development Corp., Park Ridge, NJ, 1968, p. 1.
Khan, R., The Chemistry of Sucrose, Adv. Carbohydr. Chem. Biochem. 33:268 (1976).
Allen, D.K. and B.Y. Tao, Carbohydrate-Alkyl Ester Derivatives as Biosurfactants, J. Surfact. Deterg. 2:383 (1999).
Lehrfeld, A., Separation of Some Perbenzoylated Carbohydrates by High Performance Liquid Chromatography, J. Chromatogr. 120:141 (1976).
Scholnick, F., M.K. Sucharski, and W.M. Linfield, Lactose-Derived Surfactants (I) Fatty Esters of Lactose, J. Am. Oil Chem. soc. 51:8 (1974).
Nishikawa, Y., K. Yoshimoto, M. Nishijima, F. Fukuoka, and T. Ikekawa, Chemical and Biochemical Studies on Carbohydrate Esters IX. Antitumor Effects of Selectively Fatty Acylated Products of Maltose, Chem. Pharm. Bull. 29:505 (1981).
Wing, R.E., and J.N. BeMiller, Qualitative Thin-Layer Chromatography, in Methods in Carbohydrate Chemistry, edited by R.L. Whistler and J.N. BeMiller, Academic Press, New York, 1972, Vol. 6, p. 42.
Whistler, R.L., L.W. Doner, and M. Kosik, Other Esters, in Methods in Carbohydrate Chemistry, edited by R.L. Whistler and J.N. BeMiller, Academic Press, New York, 1972, Vol. 6, p. 411
Sarney, D.B., M.J. Barnard, M. Virto, and E.N. Vulfson, Enzymatic Synthesis of Sorbitan Esters Using a Low-Boiling-Point Azeotrope as a Reaction Solvent, Biotechnol. Bioeng. 54:351 (1997).
Dische, Z., Color Reactions Based on the Reducing Properties of Sugars, in Methods in Carbohydrate Chemistry, edited by R.L. Whistler and W.L. Wolfrom, Academic Press, New York, 1962, Vol. 1, p. 513.
Padday J.F., and D.R. Russell, The Measurement of the Surface Tension of Pure Liquids and Solutions, J. Colloid Sci. 15:503 (1960).
Furlong, D.N., P.A. Freeman, I.M. Metcalfe, and L.R. White, Wall Effects in DuNouy Ring Tensiometry, J. Chem. Soc., Faraday Trans. I, 79:1701 (1983).
Rosen, M.J., Purification of Surfactants for Studies of Their Fundamental Surface Properties, J. Colloid. Interface Sci. 79:587 (1981).
Mankowich A.M., The Energetics of Surfactant Adsorption at the Air-Water Interface. J. Am. Oil Chem. Soc. 42:615 (1966).
Griffin, W.C., Classification of Surface Active Agents by “HLB,” J. Soc. Cosmet. Chem. 1:311 (1949).
American Standard for Testing and Materials E 1269-95, Annual Book of ASTM Standards. 14.02:787 (1995).
Allen, D.K., Development of Maltose Fatty Acid Esters as Biosurfactants, M.S. Thesis, Purdue University, West Lafayette, Indiana, 1999, 169 pp.
Sadtler Research Laboratories, Inc., Nuclear Magnetic Resonance Spectra, Sadtler Research Laboratories, Philadelphia, 1978.
Bollenback, G.N., and F.W. Parrish, Selective Esterification of Methyl α-d-Glucopyranoside, Carbohydr. Res. 17:431 (1971).
Whistler, R.L., and H.J. Roberts, Distribution of Formyl Groups in Amylose Monoformate, J. Am. Chem. Soc. 81:4427 (1959).
Lemieux, R.U., and A.G. McInnes, The Composition of the Sucrose Monomyristate Prepared by Transesterification, Can. J. Chem. 40:2394 (1962).
Plusquellec, D., and K. Baczko, Sugar Chemistry Without Protecting Groups: A Novel Regioselective Synthesis of 6-O-Acyl-d-glucopyranoses and Methyl-6-O-α-d-glucopyranosides, Tetrahedron Lett. 28:3809 (1987).
Griffin, W.C., Calculation of HLB Values of Non-ionic Surfactants, J. Soc. Cosmet. Chem. 5:249 (1954).
Wachs, Von W., and S. Hayano, Uber die kritische Micellkonzentration (CMC) von Fettsauremonoestern der Saccharose und ihre Beziehung zum HLB-Wert, Kolloid Z. Z. Polym. 181:139 (1961).
Soderberg, I., C.J. Drummond, D.N. Furlong, S. Godkin, and B. Matthews, Non-ionic Sugar-based Surfactants: Self-Assembly and Air/Water Interfacial Activity, Colloids Surf. A: Physicochem. Eng. Aspects. 102:91 (1995).
Drummond, C.J., G.G. Warr, and F. Grieser, Surface Properties and Micellar Interfacial Microenvironment of n-Dodecyl β-d-maltoside, J. Phys. Chem. 89:2103 (1985).
Shinoda, K., T. Yamaguchi, and R. Hori, The Surface Tension and the Critical Micelle Concentration in Aqueous Solution of β-d-Alkyl Glucosides and Their Mixtures, Bull. Chem. Soc. Jpn. 34:237 (1961).
Osipow, L.I., F.D. Snell, and J. Hickson, Surface Chemistry of Alkyl Esters of Sucrose, in Proceedings of the Second International Congress of Surface Activity, Vol. 1, Gas/Liquid and Liquid/Liquid Interface, edited by L.I. Osipow, F.D. Snell, and J. Hickson, Butterworths, London, 1957, p. 56.
Steigman, J., and N. Shane, Micelle Formation in Concentrated Sulfuric Acid as Solvent, J. Phys. Chem. 69:968 (1965).
Evans, H.C., Alkyl Sulphates. Part I. Critical Micelle Concentrations of the Sodium Salts, J. Chem. Soc. 579 (1956).
Matsumura, S., K. Ismai, S. Yoshikawa, D. Dawada, and T. Uchiburi, Surface Activities, Biodegradability, and Antimicrobial Properties of n-Alkyl Glucosides, Mannosides, and Galactosides, J. Am. Oil Chem. Soc. 67:996 (1990).
Beckett, A.H., and R.J. Woodward, Surface-Active Betaines: N-Alkyl-N,N-dimethylglycines and Their Critical Micelle Concentrations. J. Pharm. Pharmacol. 15:422 (1963).
Molyneux, P., C.T. Rhodes, and J. Swarbrick, Thermodynamics of Micellization of N-Alkyl Betaines, Trans. Faraday Soc. 61:1043 (1965).
Mitsubishi-Kagaku Foods Corporation, Physical Properties of Sugar Esters, http://www.mfc.co.jp (accessed June 1998).
Adam, N.K., The Physics and Chemistry of Surfaces, edited by N.K. Adam, Oxford University Press, London, 1941, p. 363.
Langmuir, I., The Constitution and Fundamental Properties of Solids and Liquids. II. Liquids, J. Am. Chem. Soc. 39:1848 (1917).
Klevens, H.B., Structure and Aggregation in Dilute Solutions of Surface Active Agents, J. Am. Oil Chem. Soc. 30:74 (1953).
Benson, S.W. Thermochemical Kinetics, Wiley, New York, 1968, p. 18.
Rihani, D.N., and L.K. Doraiswamy, Estimation of Heat Capacity of Organic Compounds from Group Contributions, Ind. Eng. Chem. Fundam. 4:17 (1965).
Roos, Y., Melting and Glass Transitions of Low Molecular Weight Carbohydrates, Carbohydr. Res. 238:39 (1993).
Briggner, L.F., and I. Wadso, Heat Capacities of Maltose, Maltotriose, Maltotetrose and α-, β-, and γ-Cyclodextrin in the Solid State and in Dilute Aqueous Solution, J. Chem. Thermodyn. 22:1067 (1990).
Kawaizumi, F., N. Nishio, H. Nomura, and Y. Miyahara, Heat-Capacity Measurements of Aqueous Solutions of Mono-, Di-, and Trisaccharides Using an Isoperibol Twin Calorimeter, J. Chem. Thermodyn. 13:89 (1981).
Larsson, K., Solid State Behaviour, in Lipids—Molecular Organization, Physical Functions and Technical Applications, edited by K. Larsson, Oily Press, Dundee, Scotland, 1994, p. 27.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Allen, D.K., Tao, B.Y. Synthesis and characterization of maltose fatty acid monoesters as biosurfactants. J Surfact Deterg 5, 245–255 (2002). https://doi.org/10.1007/s11743-002-0224-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11743-002-0224-y