Abstract
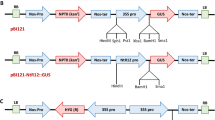
Genomic DNA for Jatropha curcas ribosome inactivating protein (JRIP) was cloned from total DNA of its leaves by polymerase chain reaction (PCR). The no intron character was confirmed. The plant expression vector pBI121-JRIP was constructed by inserting the JRIP gene into pBI121 plasmid. The recombinant Agrobacterium EHA105 strain harboring pBU21-JRIP was constructed by conducting pBI121-JRIP to strain EHA 105. PCR and Southern blotting were carried out, and the results proved that the JRIP gene was integrated into tobacco genome. It might provide a new material for disease resistance tobacco species breeding.
Similar content being viewed by others
References
Hong Y, Saunder K, Hartley MR, et al. Resistance to geminivirus infection by virus-induced expression of dian-thin in transgenic plants [J]. Virology, 1996, 220(1):119–127.
Desvoyes B, Poyet J L, Schlick J L, et al. Identification of a biological inactive complex form of pokeweed antivirl protein[J]. FEBS Letters, 1997, 410: 303–308.
Claude P S. Antifungal proteins [J]. Applied and Environmental Microbiology, 2001, 7: 2 883–2 894.
Banzet N, Latorse M P, Bulet P, et al. Expression of insect cystein-rich antifungal peptides in transgenic tobacco enhances resistance to a fungal disease [J]. Plant Science, 2002, 162: 995–1006.
Logemann J, Jach G, Tommerup H, et al. Expression of a barley ribosome-inactvating protein leads to increased fungal protection in transgenic tobacco plants [J]. Biotechnology, 1992, 10: 305–308.
Lodge J K, Kaniewski W K, Turner N E. Broad spectrum virus resistance in transgenic plants expressioning pokeweed antiviral protein [j]. Proc. Natl. Acad. Sci., USA, 1993, 90: 7 089–7 093.
Wang P, Zoubenko O, Turner N E. Reduced toxicity and broad spectrum resistance to viral and fungal infection in transgenic plants expressing pokeweed antiviral protein II [J]. Plant Mol. Biol., 1998, 38: 957–964.
Kim J K, Duan X, Wu R, et al. Molecular and genetic analysis of transgenic rice plants expressing the maize ribosome-inactivating protein b-32 gene and the herbicide resistance bar gene [J]. Molecular Breeding, 1999, 5: 85–94.
Chen Y, Peumans W J, Els J M, et al. The Sambucus nigra type-2 ribosome-inactivating protein SNA-IP exhibits in planta antiviral activity in transgenic tobacco [J]. FEBS Letters, 2002, 516: 27–30.
Rajesh K, Karen A M, Abhaya M D, et al. Expression of recombinant trichosanthin, a ribosome-inactivating protein transgenic tobacco [J]. Journal of Biotechnology, 2002, 97: 69–88.
Lin J, Chen Y, Xu Y, et al. Clong and expression of JRIP, a ribosome-inactivating protein from the seeds of Jatropha curcas [J]. Acta Botanica Sinica, 2003, 45(7): 858–863.
Lin J, Yan F, Tang L, et al. Antitumor effects of curcin from seeds of Jatropha curcas [J]. Acta Pharmacologica Sinica, 2003, 24(3): 241–246.
Wei Q, Liao Y, Zhou L J, et al. Study on antifungal activity of JRIP from the seeds of Jatropha curcas [J]. Chinese Journal of Oil Crop Sciences, 2004, 26(3): 71–75 (in Chinese).
Roger S D, Bendich A J. Extraction of DNA from Plant Tissues [A]. Plant Molecular Biology Manual, Gelvin S B, Schilperoot R A, Verma DPS, eds., Kluwer Academic Publishers, Dordrecht, 1988, A6: 1–10.
Sambrook J, Pritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual [M]. 2nd ed, Cold Spring Harbor Laboratory Press, New York, 1989.
Horsch R B, Fry J E, Hoffman N L. A simple and rapid method for transferring genes into plants [J]. Science, 1985, 227: 1229–1231.
Juan L, La Y X, Zhou X W, et al. Cloning and characterization of a curcin gene encoding a ribosome inactivating protein from Jatropha curcas [J]. DNA Sequence, 2003, 14(4): 311–317.
Ld Q L, Iiu D W, Gao X R, et al. Cloning of cDNA encoding Choline Monooxygenase from Suaeda liaotungensis and salt tolerance of transgenic tobacco [J]. Acta Botanic Sinica, 2003: 45(2): 242–247.
Zhang J X, Wang Y P, Sheng S H, et al. Transformation of Pea lectin gene and Parasponia Haemoglobin gene into rice and their expressions [J]. Acta Botanic Sinica, 2001, 43 (3): 267–274.
Chi Y. Tobacco transformation of Soubean Beta-l,3-glu-canase gene and disease resistance assay [J]. Journal of Dalian University, 2001, 22(6): 37–41 (in Chinese).
Author information
Authors and Affiliations
Additional information
Project supported by Tenth Five Years Key Program Foundation of the State Science and Technology Commission of China (Grant No. 2002BA901A15)
About this article
Cite this article
Luo, Yy., Wei, Q., Huang, Mx. et al. Isolation of a genomic DNA for Jatropha curcas ribosome inactivating protein and its tobacco transformation. J. of Shanghai Univ. 10, 461–464 (2006). https://doi.org/10.1007/s11741-006-0092-8
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11741-006-0092-8