Abstract
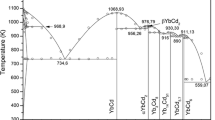
From the measured phase diagram data and experimental thermochemical properties, the DyCl3-KCl and DyCl3-CaCl2, phase diagrams were optimized and calculated by the CALPHAD technique. The Gibbs energies of liquid phase in the two systems has been optimized and calculated by new modified quasi-chemical model in the pair-approximation for short-range ordering, and a series of thermodynamic functions has also been optimized based on an interactive computer-assisted analysis. The results showed that the calculated phase diagrams and thermodynamic data were self-consistent.
Similar content being viewed by others
References
Zhang Jing, Sun Yi-min, Guan Min-yun, et al. Thermo-dynamic optimization of the CeCl3-AECl2 (AE=Mg, Ca, Sr, Ba) phase diagrams [J]. CALPHAD, 2003, 27: 305–308.
Sun Yi-min, Ye Xin-yu, Wang Yu, et al. Optimization and calculation of the NdCl3-MCl (M=Li, Na, K, Rb, Cs) phase diagrams[J]. CALPHAD, 2004, 28(2): 109–114.
Ye Xin-yu, Sun Yi-min, Wang Yu, et al. Optimization and calculation of the LaBr3-MBr (M=Na, K, Rb, Cs) phase diagrams[J]. CALPHAD, 2004, 28(2): 147–151.
Korshunov B G, Drobot D V. Reaction of the gadolium and dysprosium chlorides with sodium and potassiam Chloride in a melt [J]. Zh. Neorgan. Khim, 1965, 10(4): 939.
Blachnik R, Selle D. Thermo-chemistry of alkai chloride lanthanide(III) chlorides [J]. Z. Anorg. Attg. Chem., 1979, 454: 90–98.
Mochinaga J, Ohtani H, Igarashi K. Phase diagram of ternary dysprosium trichloride-potassium chloride-sodium chloride systems [J]. Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 1981, 49: 19.
Seifert H J, Kraemer R. Ternary chlorides in the system ACl-DyCl3(A=K, Rb, Cs)[J]. Z. Anorg. Allg. Chem., 1994, 620:1543–1548.
Seifert H J, Sandrock J, Uebach J. Thermochemical and structural investigation on the systems NaCl/TbCl; and DyCl3/NaCl [J]. Acta Chem. Scand, 1995, 49: 653.
Hatem G, Gaune-Escard M. Modeling and assessment of the DyCl3-KCl system[J]. Thermochim Acta, 1997, 293: 137–142.
Gaune-Escard M, Rycerz L, Bogacz A. Enthalpies of mixing in the DyCl3-NaCl, DyCl3-KCl and DyCl3-PrCl3, liquid systems [J]. J. Alloys Compounds, 1994, 204: 185–188.
Xing Xian-ran, Dai Shu-yu, Zhu Zhen-qi, et al. Thermo-dynamic re-optimization of the DyCl3-MCl n system [J]. Thermochim Acta, 2002, 383: 31–35.
Zheng Chao-gui, Zhao Zhong-dong, Wang Ci-qiang. The study of DyCl3-MCl n system [J]. Rare Metals, 1994, 18(4): 262–265, 269 (in Chinese).
Pelton A D, Degterov S A, Eriksson G, et al. The modified quasichemical model I-binary solutions [J]. Metall. Mater. Trans., 2000, 31B: 651.
Dinsdale A T. SGTE data for pure elements[J]. CALPHAD, 1991, 15: 317–425.
Bale C W, Pelton A D, Thompson WT. F*A*C*T-Facility for the Analysis of Chemical Thermodynamics, User’s Guide and Supplement[W/OL]. McGill University and Ecole Polytechnique, Montreal, http://www.crct. Polymtl.ca., 1999.
Guggenheim E A. The statistical mechanics of regular solutions [J]. Proc. Roy. Soc., 1935, A148: 304–312.
Fowler R H, Guggenheim E A. Statistical Thermodynamics[M]. Cambridge University, 1939, 350.
Bale C W, Pelton A D. Optimization of binary thermidynamic and phase diagram data [J]. Metall. Trans., 1983, 14B: 77.
Pelton A D, Blander M. Analysis and predictions of the thermodynamic properties of multicomponent silicates [A]. Proc. AIME Symposium on Molten Salts and Slags [C]. 1984, 281.
Pelton A D, Blander M. Thermodynamic analysis of ordered liquid solutions by a modified quasichemical approach application to silicate slags [J]. Metall. Trans., 1986, 17B: 805–815.
Blander M, Pelton A D. Thermodynamic analysis of binary liquid silicates and prediction of ternary solution properties by modified quasichemical equations [J]. Geochim. Cosmochim. Acta, 1987, 51: 85–95.
Buchel D, Seifert H J. Interaction of thulium, ytterbium (III) and lutetium chlorides with sodium chloride [J]. J. Therm. Anal. Cal., 1999, 57: 203.
Zheng C G, Seifert H J. Ternary chlorides in the systems ACl/TmCl3(A=Cs, Rb, K) [J]. J. Solid State Chemistry, 1998, 135: 127–131.
Margules M, Sitz ber Aksd. Wiss. Wien.[M], 1985, 1234.
Seifert H J, Sandrock J. Ternary chlorides in the system ACl/EuCl3(A=Na∼Cs)[J]. Z. Anorg. Allg. Chem., 1990, 587: 110–118.
Seifert H J, Sandrock J, Thiel G. Ternary chlorides in the system ACl/GdCl3 (A=Na∼Cs) [J]. Z. Anorg. Allg. Chem., 1990, 589: 307–318.
Zheng Chao-gui, Ye Yu-pu. Phase diagram of the PrCl2-SrCl2-CaCl2, system [A]. The Fifth International Symposium on Phase Diagram[C]. 1983, 3: 18.
Zheng Chao-gui, Bao Cheng-lin, Qiao Zhi-yu, et al. The phase of the PrCl3-SrCl2-CaCl2[J]. Journal of Metals, 1992, 28(8): B333.
Author information
Authors and Affiliations
Additional information
Project supported by Key Item Faundation of Anhui Municipal Commission of Education (Grant No. 2005KJ016ZD)
About this article
Cite this article
Yao, Yx., Zhang, Jk., Ma, Zs. et al. Thermodynamic optimization of the DyCl3-KCl and DyCl3CaCl2 systems. J. of Shanghai Univ. 10, 547–552 (2006). https://doi.org/10.1007/s11741-006-0055-0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/s11741-006-0055-0