Abstract
Background
Pulmonary embolism (PE) is the third-leading cause of cardiovascular death in the United States, and several studies suggest PE shows seasonal variation. Variation in monthly PE diagnosis may be due to pathophysiologic factors or confounding/bias. However, severe PE may be less prone to diagnostic bias. To address this gap, we analyzed two registries from 1/2013–12/2018 with the aim of describing temporal trends in PE diagnosis and severity.
Methods
We performed a retrospective analysis of two existing databases containing: (1) consecutive patients diagnosed with PE in the emergency departments (EDs) of two large, urban teaching hospitals, and (2) severe PEs requiring PE Response Team (PERT) activation at one of the above hospitals. The primary outcome was to assess variation in PE diagnosis and severity by calendar month. Separate analysis of these two databases sought to control for workup bias by trainee experience across the academic year. One-way ANOVA and Poisson regression were performed to assess for cyclical variation across calendar months, using Stata v16.1.
Results
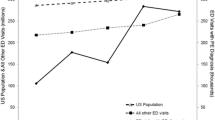
The PE diagnosis database contained 1324 patients over 36 months. One-way ANOVA did not reveal a statistically significant (p = 0.713) association between calendar month and PE number. The PERT activation database contained 1082 patients over 72 months. One-way ANOVA revealed a statistically significant (p = 0.024) association between calendar month and activations, repeated year-on-year.
Conclusion
Our results indicate correlation between calendar month and PERT activation; however, this pattern was not observed for PE diagnoses. This finding warrants further investigation into the causes of calendar month variation of PERT activations.


Similar content being viewed by others
Data availability
Requests for access to data can be sent by email to Christopher Kabrhel MD MPH at ckabrhel@mgh.harvard.edu.
References
Marti-Soler H, Gonseth S, Gubelmann C, Stringhini S, Bovet P, Chen P-C et al (2014) Seasonal variation of overall and cardiovascular mortality: a study in 19 countries from different geographic locations. PLoS ONE 9(11):e113500
Manfredini R, Gallerani M, Boari B, Salmi R, Mehta RH (2004) Seasonal variation in onset of pulmonary embolism is independent of patients’ underlying risk comorbid conditions. Clin Appl Thromb Hemost 10(1):39–43
Gallerani M, Boari B, Smolensky MH, Salmi R, Fabbri D, Contato E et al (2007) Seasonal variation in occurrence of pulmonary embolism: analysis of the database of the Emilia-Romagna region. Italy Chronobiol Int 24(1):143–160
Guijarro R, Trujillo-Santos J, Bernal-Lopez MR, De Miguel-Díez J, Villalobos A, Salazar C et al (2015) Trend and seasonality in hospitalizations for pulmonary embolism: a time-series analysis. J Thromb Haemost 13(1):23–30
Hong J, Lee JH, Lee JY, Lee JO, Choi WI, Ahn S et al (2020) Prominent seasonal variation in pulmonary embolism than deep vein thrombosis incidence: a Korean venous thrombosis epidemiology study. Korean J Intern Med 35(3):682–691
Masotti L, Ceccarelli E, Forconi S, Cappelli R (2005) Seasonal variations of pulmonary embolism in hospitalized patients. Resp Med 99(11):1469–1473
Skajaa N, Horvath-Puho E, Adelborg K, Prandoni P, Rothman KJ, Sorensen HT (2019) Venous thromboembolism in Denmark: seasonality in occurrence and mortality. TH Open 3(2):e171–e179
Olié V, Bonaldi C (2017) Pulmonary embolism: does the seasonal effect depend on age? A 12-year nationwide analysis of hospitalization and mortality. Thromb Res 150:96–100
Zhao H, Li Y, Wu M, Ren W, Ji C, Miao H et al (2020) Seasonal variation in the frequency of venous thromboembolism: an updated result of a meta-analysis and systemic review. Phlebol J Venous Dis 35(7):480–494
Perincek G, Hatipoglu ON, Tabakoglu E, Avci S (2018) Relation of the frequency and mortality of pulmonary thromboembolism with meteorological parameters. Acta Biomed 89(3):370–377
Stein PD, Kayali F, Olson RE (2004) Analysis of occurrence of venous thromboembolic disease in the four seasons. Am J Cardiol 93(4):511–513
Fares A (2013) Winter cardiovascular diseases phenomenon. N Am J Med Sci 5(4):266–279
Pell JP (1999) Seasonal variations in coronary heart disease. QJM 92(12):689–696
Hilbe J (2011) Negative binomial regression, 2nd edn. Cambridge University Press, Cambridge, UK, p 553
Mitchell MN (2012) Interpreting and visualizing regression models using Stata, 1st edn. Stata Press, College Station, TX
Gallerani M, Manfredini R, Ricci L, Grandi E, Cappato R, Calò G et al (1992) Sudden death from pulmonary thromboembolism: Chronobiological aspects. Eur Heart J 13(5):661–665
Bounameaux H, Hicklin L, Desmarais S (1996) Seasonal variation in deep vein thrombosis. BMJ 312(7026):284–285
Garriga A, Sempere-Rubio N, Molina-Prados MJ, Faubel R (2021) Impact of seasonality on physical activity: a systematic review. Int J of Environ Res Public Health 19(1):2
Van Bakel BMA, Bakker EA, De Vries F, Thijssen DHJ, Eijsvogels TMH (2021) Changes in physical activity and sedentary behaviour in cardiovascular disease patients during the COVID-19 lockdown. Int J of Environ Res Public Health 18(22):11929
Hardin JW, Hilbe JM (2018) Generalized linear models and extensions, 4th edn. Stata Press, College Station, TX
Funding
Dr. Kabrhel reports receiving grant support (paid to his institution) from Diagnostica Stago, Grifols. Dr. Kabrhel reports consulting for Abbott, BMS, Boston Scientific.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A: methodology details
Assessing normality of HRPE: monthly number of HRPEs
The null hypothesis of non-normality was rejected (Shapiro–Wilk p = 0.919). Quantile-normal plotting also suggested normality of the distribution.
Assessing appropriateness of one-way ANOVA for HRPE assessment
One-way ANOVA was confirmed as reasonable for assessing the calendar month data, by Bartlett’s test. The equal-variances test p was 0.136, corresponding to a failure to reject the H0 of equal variance.
Appendix B: extended results
One-way ANOVA results
See Tables 2, 3, 4, 5, 6, 7, 8.
The ANOVA was accompanied by a non-significant Bartlett’s test, with p = 0.136. This translates into a non-rejection of the H0 of equal variances. Thus Bartlett’s test was consistent with acceptability for use of the one-way ANOVA for the HRPE-month assessment.
Incidence rate ratios of each calendar month vs. severe PE nadir and peak months
IRRs were calculated for each calendar month compared to (1) severe PE nadir month of May, and (2) severe PE peak month of November.
Plotting of HRPEs over the entire study period (72 months)
The initial plot of HRPEs was executed over the 72 month study period, to provide an overall look at whether there were annual repeating patterns. This plotting was executed using a low bandwidth (0.1), which entailed a trade-off of “less smoothing” in favor of maximizing sensitivity for patterns of variability.
Locally weighted scatterplot smoothing trend lines for HRPEs in each calendar month
Because of the importance of the lowess trend line to represent the within-year seasonality of HRPE, the trend lines for the bandwidth settings above and below the selected bandwidth (of 0.7) are shown to demonstrate lack of dependence of the overall curve shape, on selected lowess bandwidth.
While the lowess curve had more stability at the 0.7 level (thus the selection of this bandwidth for other results), the setting of bandwidth at one level broader (0.8) or one level narrower (0.6) did not appreciably change the overall appearance of the trend line.
Poisson pairwise comparison of calendar months
Pairwise comparison of each month vs. nadir month (May)
The initial Poisson modeling step was to assess the simplest categorical model. This model was used to compare each calendar month’s HRPE n against that of the baseline month of May (i.e., the month with the lowest n). May was selected as the baseline as this resulted in positive coefficients for all other months. Results are shown in the table below.
Pairwise comparison of each month vs. peak month (November)
The above modeling approach was repeated with the selection of the highest-frequency month of November as the baseline. This resulted in negative coefficients for all other calendar months (Table 6).
Poisson modeling to evaluate seasonality
Building the Poisson model
The results for the selected cubic univariable Poisson model are shown in Table 7. Interpretation of terms in higher-order models is non-intuitive, and plain-language interpretations of the coefficients is not offered. (The coefficients are primarily useful only for sign, to convey directionality of bends in the curve). The pseudo-R2 of the model was 0.044. While pseudo-R2 is distinctly different in interpretation from linear regression’s R2, it is the case that for the exact same type of modeling on the exact same dataset a higher pseudo-R2 indicates better prediction [20].
Based on the visual inspection of the HRPE n trend line, there was no reason to believe that the linear-only or the linear + quadratic models would accurately portray HRPE trends. Assessment of these lower-order models confirmed their unsuitability.
The Poisson model that included only the linear term yielded a coefficient of 0.03 (95% CI 0.01–05) that was statistically significant (p = 0.001). However, as compared to the cubic-term Poisson model, the linear-only model’s pseudo-R2 (0.024) was reduced by nearly half. Additionally, there is the “cycling” problem that a linear-only model would call for a steady rise in HRPE from January through December, followed by a rapid large-magnitude fall after December (to get back to the cycle’s January starting point). Such a fall is not mathematically or clinically impossible, but the data indicated likelihood of a more complex cycling. There was justification to proceed with higher-order modeling.
The Poisson model that included both the linear and quadratic terms had results as shown in Table 8. Neither term was statistically significant, and the quadratic model’s pseudo-R2 was substantially less favorable than that of the cubic-term model.
Although the curve did not appear to have more than two within-year bends, a quartic model was also evaluated. All p values (linear, quadratic, cubic, quartic) were non-significant (p 0.341 or higher). There was no justification to proceed with testing further polynomials.
Assessing the cubic Poisson model for appropriateness
The Poisson model presumes that the variance is equal to the mean. Since overdispersion renders standard Poisson modeling inappropriate (e.g., by risking false identification of a non-important variable as significant), a test for dispersion should be performed to assure Poisson should not be discarded in favor of other modeling approaches (e.g., negative binomial regression).
The initial evaluation for dispersion is the Pearson dispersion statistic. This statistic was 1.13 for the cubic Poisson model. Since the statistic was near one, there was suggestion that the model was well-specified [20]. However, since the Pearson dispersion statistic did exceed one, the question of overdispersion arose. The next step was to assess formally for overdispersion.
Using Hardin and Hilbe’s regression-based test of obtaining fitted values and calculating a z score [20], the constant-only z regression had a p value of 0.720. This translated into non-rejection of the H0 of no overdispersion. Thus by this test there was no indication the data were not suitable for this study’s Poisson modeling approach. The cubic Poisson model was well-specified. This finding was reinforced by a non-significant Pearson goodness-of-fit test (p = 0.215) which was consistent with a good fit between the study data and the selected Poisson model.
Appendix B: study setting
The PERT diagnosis database included data from the EDs of Hospitals A and B. The PERT activation database included data from Hospital A.
See Table 9.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thomas, S.A., Wang, G., Giordano, N. et al. Calendar month variation in the diagnosis and severity of pulmonary embolism. Intern Emerg Med 18, 879–887 (2023). https://doi.org/10.1007/s11739-022-03180-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-022-03180-w