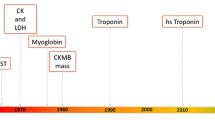
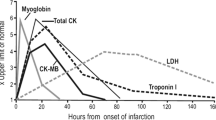
Abstract
Current diagnostic biomarkers for ACS are mainly represented by troponin I and troponin T. Dosing of these two molecules often leads to false positive results, since their plasma levels can increase in several different systemic settings. Therefore, identification of new markers able to detect patients with acute coronary syndromes is an emerging priority. On this view, many studies have been performed on different microRNAs, mitochondrial peptides, inflammatory cytokines and adhesion molecules with very promising results. Besides their introduction in screening programs, further studies are now needed in the acute setting, beyond or in association with troponin levels. This will help to better discriminate the real occurrence of an ACS in many patients accessing the emergency department for chest pain.
Similar content being viewed by others
References
Velle-Forbord T et al (2019) Circulating microRNAs as predictive biomarkers of myocardial infarction: evidence from the HUNT study. Atherosclerosis. https://doi.org/10.1016/j.atherosclerosis.2019.07.024
Long B, Long DA, Tannenbaum L, Koyfman A (2019) An emergency medicine approach to troponin elevation due to causes other than occlusion myocardial infarction. Am J Emerg Med. https://doi.org/10.1016/j.ajem.2019.12.007
Roffi M et al (2016) 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. https://doi.org/10.1093/eurheartj/ehv320
Gilardi E, Iacomini P, Marsiliani D, De Marco G, Covino M (2014) Biomarkers in the prediction and management of acute coronary syndromes: current perspectives. Res Reports Clin Cardiol. https://doi.org/10.2147/rrcc.s36294
Wang X et al (2013) Increased expression of microRNA-146a decreases myocardial ischaemia/reperfusion injury. Cardiovasc Res. https://doi.org/10.1093/cvr/cvs356
Raitoharju E et al (2011) MiR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere vascular study. Atherosclerosis. https://doi.org/10.1016/j.atherosclerosis.2011.07.020
Xue S et al (2019) Circulating miR-26a-1, miR-146a and miR-199a-1 are potential candidate biomarkers for acute myocardial infarction. Mol Med 25:1–12
Chiang MH et al (2020) miR-26a attenuates cardiac apoptosis and fibrosis by targeting ataxia–telangiectasia mutated in myocardial infarction. J Cell Physiol. https://doi.org/10.1002/jcp.29537
Wang J et al (2014) miR-499 protects cardiomyocytes from H2O2-induced apoptosis via its effects on Pdcd4 and Pacs2. RNA Biol. https://doi.org/10.4161/rna.28300
Zhang YH, He K, Shi G (2017) Effects of microRNA-499 on the inflammatory damage of endothelial cells during coronary artery disease via the targeting of PDCD4 through the NF-Κβ/TNF-α signaling pathway. Cell Physiol Biochem. https://doi.org/10.1159/000484588
Bernardo BC et al (2012) Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1206432109
Boon RA et al (2013) MicroRNA-34a regulates cardiac ageing and function. Nature. https://doi.org/10.1038/nature11919
Kumar D et al (2020) Circulatory miR-133b and miR-21 as novel biomarkers in early prediction and diagnosis of coronary artery disease. Genes (Basel) 11(2):164
Gigante B et al (2020) MicroRNA signatures predict early major coronary events in middle-aged men and women. Cell Death Dis 11:10–12
Chen Y, Ye X, Yan F (2019) MicroRNA 3113-5p is a novel marker for early cardiac ischemia/reperfusion injury. Diagn Pathol. https://doi.org/10.1186/s13000-019-0894-1
Aurora AB et al (2012) MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2+ overload and cell death. J Clin Invest. https://doi.org/10.1172/JCI59327
Hullinger TG et al (2012) Inhibition of miR-15 protects against cardiac ischemic injury. Circ Res. https://doi.org/10.1161/CIRCRESAHA.111.244442
Yang Y et al (2015) Plasma long non-coding RNA, CoroMarker, a novel biomarker for diagnosis of coronary artery disease. Clin Sci. https://doi.org/10.1042/CS20150121
Li M et al (2018) Circulating long noncoding RNA LIPCAR acts as a novel biomarker in patients with ST-segment elevation myocardial infarction. Med Sci Monit. https://doi.org/10.12659/MSM.909348
Lee C, Kim KH, Cohen P (2016) MOTS-c: a novel mitochondrial-derived peptide regulating muscle and fat metabolism. Free Radical Biol Med. https://doi.org/10.1016/j.freeradbiomed.2016.05.015
Qin Q et al (2018) Downregulation of circulating MOTS-c levels in patients with coronary endothelial dysfunction. Int J Cardiol. https://doi.org/10.1016/j.ijcard.2017.12.001
Yang Y et al (2019) The role of mitochondria-derived peptides in cardiovascular disease: recent updates. Biomed Pharmacother 117:109075
Yen K, Lee C, Mehta H, Cohen P (2012) The emerging role of the mitochondrial-derived peptide humanin in stress resistance. J Mol Endocrinol. https://doi.org/10.1530/JME-12-0203
Muzumdar RH et al (2010) Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice. Arterioscler Thromb Vasc Biol. https://doi.org/10.1161/ATVBAHA.110.205997
Hashimoto Y, Kurita M, Aiso S, Nishimoto I, Matsuoka M (2009) Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor α/WSX-1/gp130. Mol Biol Cell. https://doi.org/10.1091/mbc.E09-02-0168
Lee C, Yen K, Cohen P (2013) Humanin: a harbinger of mitochondrial-derived peptides? Trends Endocrinol Metab. https://doi.org/10.1016/j.tem.2013.01.005
Widmer RJ et al (2013) Circulating humanin levels are associated with preserved coronary endothelial function. Am J Physiol Heart Circ Physiol 304(3):H393–H397
Thummasorn S, Shinlapawittayatorn K, Chattipakorn SC, Chattipakorn N (2017) High-dose Humanin analogue applied during ischemia exerts cardioprotection against ischemia/reperfusion injury by reducing mitochondrial dysfunction. Cardiovasc Ther. https://doi.org/10.1111/1755-5922.12289
Liang J et al (2009) Myeloperoxidase (MPO) and interleukin-17 (IL-17) plasma levels are increased in patients with acute coronary syndromes. J Int Med Res. https://doi.org/10.1177/147323000903700331
Ávalos AM et al (2012) IL-17A levels increase in the infarcted region of the left ventricle in a rat model of myocardial infarction. Biol Res. https://doi.org/10.4067/S0716-97602012000200012
Liao YH et al (2012) Interleukin-17A contributes to myocardial ischemia/reperfusion injury by regulating cardiomyocyte apoptosis and neutrophil infiltration. J Am Coll Cardiol. https://doi.org/10.1016/j.jacc.2011.10.863
Xu Y et al (2020) The expression of interleukin-25 increases in human coronary artery disease and is associated with the severity of coronary stenosis. Anatol J Cardiol 23:151–159
Tajfard M et al (2017) Serum concentrations of MCP-1 and IL-6 in combination predict the presence of coronary artery disease and mortality in subjects undergoing coronary angiography. Mol Cell Biochem 435:37–45
Ridker PM et al (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. https://doi.org/10.1056/NEJMoa1707914
Schofer N, Ludwig S, Rübsamen N, Schnabel R, Lackner KJ, Ruprecht HJ, Bickel C, Landmesser U, Blankenberg S, Zeller T (2018) Prognostic impact of interleukin-1 receptor antagonist in patients with documented coronary artery disease. Int J Cardiol. 257:24–29
Buckley LF, Abbate A (2018) Interleukin-1 blockade in cardiovascular diseases: from bench to bedside. Bio Drugs 32:111–118
Zheng Z-H, Zeng X, Nie X-Y, Cheng Y-J, Liu J, Lin X-X, Yao H, Ji C-C, Chen X-M, Jun F, Su-Hua Wu (2019) Interleukin-1 blockade treatment decreasing cardiovascular risk. Clin Cardiol 42:942–951
Herder C, Heras Gala TL, Carstensen-Kirberg M, Huth C, Zierer A, Wahl S, Sudduth-Klinger J, Kuulasmaa K, Peretz D, Ligthart S, Bongaerts BWC, Dehghan A, Arfan Ikram M, Jula A, Kee F, Pietilä A, Saarela O, Zeller T, Blankenberg S, Meisinger C, Peters A, Roden M, Salomaa V, Koenig W, Thorand B (2017) Circulating levels of interleukin 1-receptor antagonist and risk of cardiovascular disease: meta-analysis of six population-based cohorts. Arterioscler Thromb Vasc Biol. 37:1222–1227
Hjort M, Eggers KM, Lindhagen L, Agewall S, Brolin EB, Collste O, Daniel M, Ekenbäck C, Frick M, Henareh L, Hofman-Bang C, Malmqvist K, Spaak J, Sörensson P, Hassan SY, Tornvall P, Lindahl B (2019) Increased inflammatory activity in patients 3 months after myocardial infarction with nonobstructive coronary arteries. Clin Chem. 65:1023–1030
Qian W, Zhao C, Li D, Dai R (2018) Mechanism of interleukin-1 receptor antagonist protection against myocardial ischaemia/reperfusion-induced injury. Arch Cardiovasc Dis 111:545–554
Hulok A et al (2014) Soluble cell adhesion molecules—does estimating sVCAM-1 and sICAM-1 concentration provide additional information about cardiovascular risk in patients with coronary artery disease? Adv Clin Exp Med 23:735–741
Liu A, Wan A, Feng A, Rui R, Zhou B (2018) ICAM-1 gene rs5498 polymorphism decreases the risk of coronary artery disease. Medicine (United States). https://doi.org/10.1097/MD.0000000000012523
Yin DL et al (2019) Association between the ICAM-1 gene polymorphism and coronary heart disease risk: a meta-analysis. Biosci Rep 39:1–7
Wang Y et al (2019) The role of OX40L and ICAM-1 in the stability of coronary atherosclerotic plaques and their relationship with sudden coronary death. BMC Cardiovasc Disord. https://doi.org/10.1186/s12872-019-1251-8
Radecke CE et al (2015) Coronary artery endothelial cells and microparticles increase expression of VCAM-1 in myocardial infarction. Thromb Haemost. https://doi.org/10.1160/TH14-02-0151
Dos Santos JC et al (2018) Relationship between circulating VCAM-1, ICAM-1, E-selectin and MMP9 and the extent of coronary lesions. Clinics. https://doi.org/10.6061/clinics/2018/e203
Ambros V (2004) The functions of animal microRNAs. Nature 431:350–355
Cheng Y et al (2010) A translational study of circulating cell-free microRNA-1 in acute myocardial infarction. Clin Sci. https://doi.org/10.1042/CS20090645
Dong S et al (2009) MicroRNA expression signature and the role of MicroRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. https://doi.org/10.1074/jbc.M109.027896
Iaconetti C, Gareri C, Polimeni A, Indolfi C (2013) Non-coding RNAs: the ‘dark matter’ of cardiovascular pathophysiology. Int J Mol Sci. https://doi.org/10.3390/ijms141019987
Ren J et al (2013) Signature of circulating MicroRNAs as potential biomarkers in vulnerable coronary artery disease. PLoS ONE. https://doi.org/10.1371/journal.pone.0080738
De Rosa S, Curcio A, Indolfi C (2014) Emerging role of micrornas in cardiovascular diseases. Circ J 78:567–575
Islas JF, Moreno-Cuevas JE (2018) A microRNA perspective on cardiovascular development and diseases: an update. Int J Mol Sci. https://doi.org/10.3390/ijms19072075
Sun T et al (2017) The role of microRNAs in myocardial infarction: from molecular mechanism to clinical application. Int J Mol Sci. https://doi.org/10.3390/ijms18040745
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest.
Statement of human and animal rights
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Piccioni, A., Valletta, F., Zanza, C. et al. Novel biomarkers to assess the risk for acute coronary syndrome: beyond troponins. Intern Emerg Med 15, 1193–1199 (2020). https://doi.org/10.1007/s11739-020-02422-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-020-02422-z