Abstract
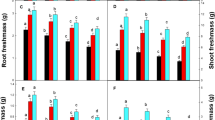
The effect of Ca on Cu toxicity in runner bean plants (Phaseolus coccineus L. cv. Piěkny Jaś) grown hydroponically in nutrient solution was studied. The toxic effect of excess Cu on plants depends on their age and Ca content in the medium. Copper applied in excess to the plants at the early phase of leaf development strongly limits the uptake of Ca ions from the nutrient solution, particularly their translocation to leaves. Increased Ca content limits the inhibitory effect of Cu on leaf growth and decreases the content of chloroplast pigments to the level approximate to that of control. At this growth stage the effect of excess Cu is at least partially connected with limited Ca transport to leaves.
At the intermediate leaf phase Cu-treated plants react slightly to changed Ca content.
At the end of the primary leaf development increased Ca concentration in the medium intensifies senescence processes induced by excess Cu. The changes are partially connected with intensified water deficit. Increased Ca content in the nutrient solution limits Cu accumulation in the individual organs of Cu-treated plants. However, Cu accumulation in leaves is not decreased at a high level of Ca. Copper generally decreases Ca content in the youngest plants, whereas in the oldest ones only in the case of a low level of Ca in the nutrient solution.
Similar content being viewed by others
Abbreviations
- Car:
-
carotenoids
- Chl:
-
chlorophyll
- PPFD:
-
photosynthetic photon flux density
- PSII:
-
photosystem II
References
Agarwal K., Sharma A., Talukder G. 1987. Copper toxicity in plant cellular systems. Nucleus, 30: 131–158.
Cunninghame M.E., Hall J.L. 1986. The effect of calcium antagonists and inhibitors of secretory processes on auxin-induced elongation and fine structure of Pisum sativum stem segments. Protoplasma, 133: 149–155.
Feldman L.J. 1981. Light-induced inhibitors from intact and cultured caps of Zea roots. Planta, 153: 471–474.
Fernandes J.C., Henriques F.S. 1991. Biochemical, physiological, and structural effects of excess copper in plants. Bot. Rev., 57: 246–273.
Ghanotakis D.F., Yocum C.F. 1990. Photosystem II and the oxygen-evolving complex. Annu. Rev. Plant Physiol. Plant Mol. Biol., 41: 255–276.
Hanson J.B. 1984. The function of calcium in plant nutrition. In: Advance in Plant Nutrition, ed. by P.B. Tinker, A. Läuchli, Praeger Scientific, New York: 149–208.
Hepler P.K. 1994. The role of calcium in cell division. Cell Calcium, 16: 322–330.
Jensén P., Adalsteinsson S. 1989. Effects of copper on active and passive Rb+ influx in roots of winter wheat. Physiol. Plant, 75: 195–200.
Krieger A., Weis E. 1993. The role of calcium in the pH-dependent control of photosystem II. Photosynth. Res., 37: 117–130.
Leopold A.C., Willing R.P. 1984. Evidence for toxicity effects of salts on membranes. In: Salinity Tolerance in Plants. Strategies for Corp. Improvements, ed. by R.C. Staples, G.H. Toenniessen, John Wiley and Sons, New York: 67–80.
Leshem Y.Y. 1984. Ca2+: calmodulin -induced and ethylene mediated membranal phospholipid catabolism as a mode of plant senescence. In: Structure, Function and Metabolism of Plant Lipids, ed. by P.A. Siegenthaler, W. Eichenberger, Elsevier, Amsterdam: 181–188.
Leshem Y.Y. 1991. Plant membrane senescence. In: Plant Signalling. Plasma Membrane, and Change of State, ed. by C. Penel, H. Greppin, Laboratory of Plant Physiology University of Geneva: 31–58.
Lichtenthaler H.K., Wellburn R.R. 1983. Determination of total carotenoids and chlorophylls a and b of extracts in different solvents. Biochem. Soc. Trans., 603: 591–592.
Mahmood T., Cochrane M.P, Vinten A.J.A., Duffus C.M. 1994. Calcium-induced modification of copper, depending upon their growth stage. Biol. Plant. (Suppl) 36: 196.
Maksymiec W., Bednara J., Baszyński T. 1995. Responses of runner bean plants to excess copper as a function of plant growth stages: Effects on morphology and structure of primary leaves and their chloroplast ultrastructure. Photosynthetica, 31: 427–435.
Maksymiec W., Baszyński. T. 1996a. Chlorophyll fluorescence in primary leaves of excess Cu-treated runner bean plants depends on their growth stages and the duration of Cu-action. J. Plant. Physiol., 149: 196–200.
Maksymiec W., Baszyński T. 1996b. Different susceptibility of runner bean plants to excess copper as a function of the growth stages of primary leaves. J. Plant. Physiol., 149: 217–221.
Maksymiec W. 1997. Effect of copper on cellular processes in higher plants. Photosynthetica, 34: 321–342.
Marschner H. 1988. Mineral nutrition of higher plants. Academic Press, London, San Diego, New York, Boston, Sydney, Tokyo, Toronto, Academic Press INC.
Ouzounidou G., Eleftheriou E.S., Karataglis S. 1992. Ecophysiological and ultrastructural effect of copper in Thlaspi ochroleucum (Cruciferae). Can J. Bot., 70: 947–957.
Ouzounidou G. 1994. Copper-induced changes on growth, metal content and photosynthetic function of Alyssum montanum L. plants. Environ. Exp. Bot., 34: 165–172.
Ouzounidou G., Symeonidis L., Babalonas D., Karataglis S. 1994. Comparative response of a copper-tolerant and copper sensitive population of Minuartia hirsuta to copper toxicity. J. Plant Physiol., 144; 109–115.
Ouzounidou, G., Moustakas M., Lannoye R. 1995. Chlorophyll fluorescence and photoacoustic characteristics in relationship to changes in chlorophyll and Ca2+ content of a Cu-tolerant Silene compacta ecotype under Cu treatment. Physiol. Plant., 93: 551–557.
Poovaiah B.W. 1987. The role of calcium and calmodulin in senescence. In: Plant Senescence: its biochemistry and physiology, ed. by W.W Tomson, E.A. Natnagel, R.C. Huffaker, American Society of Plant Physiologists, Rockville: 182–189.
Poovaiah B.W., Reddy A.S.N., McFadden J.J. 1987. Calcium mesenger system: role of protein phosphorylation and inositol phospholipids. Physiol. Plant., 69: 569–573.
Ramalho J.C., Rebelo M.C., Santos E.M. Antunes L.M., Nunes A.M. 1995. Effects of calcium deficiency on Coffea arabica. Nutrient changes and correlation of calcium levels with some photosynthetic parameters. Plant and Soil, 172: 87–96.
Yocum Ch.F. 1991. Calcium activation of photosynthetic water oxidation. Biochem. Biophys. Acta, 1059: 1–15.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maksymiec, W., Baszyński, T. The role of Ca ions in changes induced by excess Cu2+ in bean plants. Growth parameters. Acta Physiol Plant 20, 411–417 (1998). https://doi.org/10.1007/s11738-998-0028-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11738-998-0028-y