Abstract
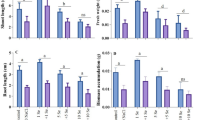
Salinity stress is continuously declining crop production throughout the world. Various strategies are in use to mitigate its effect on crop plants. The current study was performed to resolve the effects of exogenous selenium (Se) (1 μM) on the two millet varieties viz., Panicum miliaceum L. (proso millet: PM) and Setaria italica L. (foxtail millet: FM), which were subjected to salt stress (NaCl) of varying amounts. The salt treatments (50–200 mM NaCl) decreased the tolerance index (TI) of both shoots and roots and the Se application reversed the effect. The PM shoots and FM roots showed better tolerance index ranging up to 95.42% and 101%, respectively. The salt treatments decreased the biomass, relative water content (RWC) and photosynthetic pigments (PP) in a dose-dependent manner with respect to control. The Se application showed a maximum improvement in biomass (49.09% and 293.28%), RWC (14.49% and 20.42%) and PP (228.86% and 507.22%) in PM and FM, respectively, in comparison to highest salt treatment (200 mM). The salt treatments increased the membrane damage as evidenced by electrolyte leakage (EL) and thiobarbituric acid reactive species (TBARS). However, Se application showed improvement in EL (15.62% and 49.18%) and TBARS (42.34% and 34.20%) in PM and FM, respectively, in comparison to salt treatments. When comparing the two varieties for the parameters it was found that FM performed better than PM. The Se-mediated resistance of millets (PM, FM) toward salinity makes them a model for studying stress responses at different stages of growth and development. This would help screen the resistant varieties and in future, these could be utilized for cultivation on marginal lands for sustainable growth and yield. Thus, overall the application of Se in low doses offers promising potential for use in high salinity conditions.






Similar content being viewed by others
References
Ahmad R, Hussain S, Anjum MA, Khalid MF, Saqib M, ZakirI AS (2019) Oxidative stress and antioxidant defense mechanisms in plants under salt stress. In: Hasanuzzaman M, Hakeem K, Nahar K, Alharby H (eds) Plant abiotic stress tolerance. Springer, Cham, pp 191–205
Amadou I, Gounga ME, Le GW (2013) Millets: nutritional composition, some health benefits and processing—a review. Emirates J Food Agric 25:501–508
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141(2):391–396
Ashraf MA, Muhammad A (2012) Salt-induced variation in some potential physiochemical attributes of two genetically diverse spring wheat (Triticum aestivum L.) cultivars: photosynthesis and photosystem II efficiency. Pak J Bot 44(1):53–64
Ashraf MPJC, Harris PJC (2004) Potential biochemical indicators of salinity tolerance in plants. Plant Sci 166(1):3–16
Ashraf MA, Akbar A, Parveen A, Rasheed R, Hussain I, Iqbal M (2018) Phenological application of selenium differentially improves growth, oxidative defense and ion homeostasis in maize under salinity stress. Plant Physiol Biochem 123:268–280
Babitha KC, Vemanna RS, Nataraja KN, Udayakumar M (2015) Overexpression of EcbHLH57 transcription factor from Eleusine coracana L. in tobacco confers tolerance to salt, oxidative and drought stress. PLoS ONE 10(9):e0137098
Bandyopadhyay T, Muthamilarasan M, Prasad M (2017) Millets for next-generation climate-smart agriculture. Front Plant Sci 8:1266
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Anals Bot 91(2):179–194
Breznik B, Germ M, Gaberscik A, Kreft I (2005) Combined effects of elevated UV-B radiation and the addition of selenium on common (Fagopyrum esculentum Moench) and Tartary [Fagopyrum tataricum (L.) Gaertn.] buckwheat. Photosynthetica 43(4):583–589
Cakmak I, Horst WJ (1991) Effect of aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83(3):463–468
Dash M, Panda SK (2001) Salt stress-induced changes in growth and enzyme activities in germinating Phaseolus mungo seeds. Biologia Plant 44(4):587–589
Diao M, Ma L, Wang J, Cui J, Fu A, Liu HY (2014) Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing the chloroplast antioxidant defense system. J Plant Growth Reg 33(3):671–682
Djanaguiraman M, Prasad PV, Seppanen M (2010) Selenium protects sorghum leaves from oxidative damage under high-temperature stress by enhancing the antioxidant defense system. Plant Physiol Biochem 48(12):999–1007
FAOSTAT (2016) FAO Food and Agriculture Database. Statistics Division, Food and Agriculture Organization of the United Nations. https://faostat.fao.org/. Accessed 25 Nov 2016
Farooq M, Hussain M, Wakeel A, Siddique KH (2015) Salt stress in maize: effects, resistance mechanisms, and management. A review. Agron Sustain Dev 35(2):461–481
Feng R, Wei C, Tu S (2013) The roles of selenium in protecting plants against abiotic stresses. Environ Exp Bot 87:58–68
Fuller DQ (2006) A millet atlas: some identification guidance. University College London, London
Germ M, Kreft I, Osvald J (2005a) Influence of UV-B exclusion and selenium treatment on photochemical efficiency of photosystem II, yield and respiratory potential in pumpkins (Cucurbita pepo L.). Plant Physiol Biochem 43(5):445–448
Germ M, Kreftb I, Osvaldb J (2005b) Influence of UV-B exclusion and selenium treatment on photochemical efficiency of photosystem II, yield and respiratory potential in pumpkins (Cucurbita pepo L.). Plant Physiol Bioch 43:445–448
Ghoulam C, Fares K (2001) Effect of salinity on seed germination and early seedling growth of sugar beet (Beta vulgaris L.). Seed Sci Tech 29(2):357–364
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 12:909–1026
Gorai M, Ennajeh M, Khemira H, Neffati M (2010) Combined effect of NaCl-salinity and hypoxia on growth, photosynthesis, water relations and solute accumulation in Phragmitesaustralis plants. Flora-Morphol Distrib Func Ecol Plants 205(7):462–470
Habib SH, Kausar H, Saud HM (2016) Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. BioMed Res Inter 2016:6284547. https://doi.org/10.1155/2016/6284547
Habibi G, Sarvary SJ (2015) The roles of selenium in protecting lemon balm against salt stress. Iran J Plant Physiol 5:1425–1433
Hameed M, Ashraf M (2008) Physiological and biochemical adaptations of Cynodon dactylon (L.) Pers. from the Salt Range (Pakistan) to salinity stress. Flora-Morphol Distrib Funct Ecol Plants 203(8):683–694
Hartikainen H, Xue T, Piironen V (2000) Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil 225(1–2):193–200
Hasanuzzaman M, Hossain MA, Fujita M (2011) Selenium-induced up-regulation of the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biol Trace Element Res 143(3):1704–1721
Hawrylak-Nowak B (2009) Beneficial effects of exogenous selenium in cucumber seedlings subjected to salt stress. Biol Trace Element 132:259–269
Ivanova D, Zhelev Z, Aoki I, Bakalova R, Higashi T (2016) Overproduction of reactive oxygen species-obligatory or not for induction of apoptosis by anticancer drugs. Chin J Cancer Res 28:38
Jamil A, Riaz S, Ashraf M, Foolad MR (2011) Gene expression profiling of plants under salt stress. Crit Rev Plant Sci 30(5):435–458
Jiang C, Zu C, Lu D, Zheng Q, Shen J, Wang H, Li D (2017) Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci Rep 7:42039
Kaur P, Purewal SS, Sandhu KS, Kaur M, Salar RK (2018) Millets: a cereal grain with potent antioxidants and health benefits. J Food Measur Character 13(1):793–806
Kole C, Muthamilarasan M, Henry R, Edwards D, Sharma R, Abberton M (2015) Application of genomics-assisted breeding for generation of climate resilient crops: progress and prospects. Front Plant Sci 6:563
Kong L, Wang M, Bi D (2005) Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the growth of sorrel seedlings under salt stress. Plant Growth Reg 45(2):155–163
Kumar M, Bijo AJ, Baghel RS, Reddy CRK, Jha B (2012) Selenium and spermine alleviate cadmium-induced toxicity in the red seaweed Gracilaria dura by regulating antioxidants and DNA methylation. Plant Physiol Biochem 51:129–138
Liang W, Ma X, Wan P, Liu L (2018) Plant salt-tolerance mechanism: a review. Biochem Biophy Res Comm 495(1):286–291
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Lu K, Ding W, Zhu S, Jiang D (2016) Salt-induced difference between Glycine cyrtoloba and G. max in anti-oxidative ability and K+ vs. Na+ selective accumulation. Crop J 4:129–138
Mancinelli AL (1990) Interaction between light quality and light quantity in the photoregulation of anthocyanin production. Plant Physiol 92(4):1191–1195
Mittal S, Kumari N, Sharma V (2012) Differential response of salt stress on Brassica juncea: photosynthetic performance, pigment, proline, D1, and antioxidant enzymes. Plant Physiol Biochem 54:17–26
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Nawaz F, Naeem M, Ashraf MY, Tahir MN, Zulfiqar B, Salahuddin M, Aslam M (2016) Selenium supplementation affects physiological and biochemical processes to improve fodder yield and quality of maize (Zea mays L.) under water deficit conditions. Front Plant Sci 7:1438
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60(3):324–349
Pilon-Smits EA, Quinn CF, Tapken W, Malagoli M, Schiavon M (2009) Physiological functions of beneficial elements. Curr Opin Plant Biol 12(3):267–274
Rehman S, Abbas G, Shahid M, Saqib M, Farooq ABU, Hussain M (2019) Effect of salinity on cadmium tolerance, ionic homeostasis and oxidative stress responses in conocarpus exposed to cadmium stress: Implications for phytoremediation. Ecotoxicol Environ Saf 171:146–153
Sairam RK, Srivastava GC (2002) Changes in antioxidant activity in subcellular fractions of tolerant and susceptible wheat genotypes in response to long term salt stress. Plant Sci 162(6):897–904
Sapeta H, Costa JM, Lourenco T, Maroco J, Van der Linde P, Oliveira MM (2013) Drought stress response in Jatropha curcas: growth and physiology. Environ Exp Bot 85:76–84
Sattar A (2017) Separate and combined effects of silicon and selenium on salt tolerance of wheat plants. Rus J Plant Physiol 64(3):341–348
Shahbaz M, Ashraf M (2013) Improving salinity tolerance in cereals. Crit Rev Plant Sci 32(4):237–249
Shahbaz M, Ashraf MA-Q, Harris PJC (2012) Salt tolerance in selected vegetable crops. Crit Rev Plant Sci 31(4):303–320
Shahid SA, Zaman M, Heng L (2018) Soil salinity: historical perspectives and a world overview of the problem. Guideline for salinity assessment mitigation and adaptation using nuclear and related techniques. Springer, Cham, pp 43–53
Shi D, Sheng Y (2005) Effect of various salt–alkaline mixed stress conditions on sunflower seedlings and analysis of their stress factors. Environ Exp Bot 54(1):8–21
Stepien P, Klobus G (2005) Antioxidant defense in the leaves of C3 and C4 plants under salinity stress. Physiol Plant 125:31–40
Subramanyam K, Du Laing G, Van Damme EJ (2019) Sodium selenate treatment using a combination of seed priming and foliar spray alleviates salinity stress in rice. Front Plant Sci 10:116
Tabot PT, Adams JB (2013) Early responses of Bassia diffusa (Thunb.) Kuntze to submergence for different salinity treatments. S Afr J Bot 84:19–29
Taïbi K, Taïbi F, Abderrahim LA, Ennajah A, BelkhodjaM MJM (2016) Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S Afr J Bot 105:306–312
Van RJM, Hekimi S (2012) Superoxide dismutase is dispensable for normal animal lifespan. Proc Natl Acad Sci 109:5785–5790
Wang CQ (2011) Water-stress mitigation by selenium in Trifolium repens L. J Plant Nutr Soil Sci 174(2):276–282
Wilkins DA (1957) A technique for the measurement of lead tolerance in plants. Nature 180(4575):37
Winterbourn CC (2019) Reactive oxygen species in biological systems. In: Niki E (ed) Vitamin E, chemistry and Nutritional benefits, RSC, pp 98–117
Xue T, Hartikainen H, Piironen V (2001) Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil 237(1):55–61
Yang F, Xiao X, Zhang S, Korpelainen H, Li C (2009) Salt stress responses in Populus cathayana Rehder. Plant Sci 176(5):669–677
Yang Y, Guo Y (2018) Elucidating the molecular mechanisms mediating plant salt stress responses. New phytol 217(2):523–539
Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6(5):441–445
Zhu JK (2007) Plant salt stress. In: Encyclopedia of life sciences. Wiley. www.els.net. Accessed 3 Feb 2020
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by M. Prasad.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rasool, A., Shah, W.H., Tahir, I. et al. Exogenous application of selenium (Se) mitigates NaCl stress in proso and foxtail millets by improving their growth, physiology and biochemical parameters. Acta Physiol Plant 42, 116 (2020). https://doi.org/10.1007/s11738-020-03109-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-020-03109-w