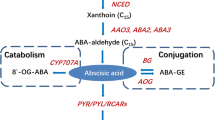
Abstract
Phytohormones regulate numerous aspects of plant growth and development. Green-mature banana fruit were treated with deionized water (control), abscisic acid (ABA), indole-3-acetic acid (IAA) and ABA + IAA, respectively, to investigate the role of ABA and IAA in fruit ripening. Results showed that ABA accelerated fruit ripening, but IAA delayed the process. However, treatment of ABA + IAA showed little difference in fruit color and firmness. The acceleration of ABA and delay of IAA on banana ripening process seems to be neutralized by ABA + IAA. Digital gene expression revealed that ABA + IAA treated fruit maintained the similar color phenotype with the control by regulating the expression of chlorophyll degradation-related gene PaO (GSMUA_Achr6G25590_001), and carotenoid biosynthesis-related genes DXR (GSMUA_Achr3G20790_001) and PSY (GSMUA_Achr2G12480_001, GSMUA_Achr4G17270_001, GSMUA_Achr4G17290_001). Moreover, ABA + IAA treated fruit maintained the similar softening phenotype with the control by adjusting the expression of pectin degradation-related genes PME (GSMUA_Achr3G05740_001) and PL (GSMUA_Achr6G28160_001, GSMUA_Achr7G04580_001). ABA + IAA treatment nearly abolished the action of individual ABA or IAA through equilibrating the expression of specific genes involved in chlorophyll degradation, carotenoid biosynthesis and pectin degradation pathways in the postharvest ripening of banana. The interaction between ABA and IAA might exercise as an antagonistic mechanism of neutralizing the specific gene expression either induced by ABA or reduced by IAA in the postharvest ripening of banana.




Similar content being viewed by others
References
Albersheim P, Darvill AG, O’Neill MA, Schols HA, Voragen AGJ (1996) An hypothesis: the same six polysaccharides are components of the primary cell walls of all higher plants. Pectins and pectinases. Elsevier Sciences B.V., Amsterdam, pp 47–53
Bapat VA, Trivedi PK, Ghosh A, Sane VA, Ganapathi TR, Nath P (2010) Ripening of fleshy fruit: molecular insight and the role of ethylene. Biotechnol Adv 28:94–107
Bottcher C, Boss PK, Davies C (2012) Delaying riesling grape berry ripening with a synthetic auxin affects malic acid metabolism and sugar accumulation, and alters wine sensory characters. Funct Plant Biol 39(9):745–753
Cação SM, Leite TF, Budzinski IG, dos Santos TB, Scholz MB, Carpentieri-Pipolo V, Domingues DS, Vieira LG, Pereira LF (2012) Gene expression and enzymatic activity of pectin methylesterase during fruit development and ripening in Coffea arabica L. Genet Mol Res 11(3):3186–3197
Cao J (2012) The pectin lyases in Arabidopsis thaliana: evolution, selection and expression profiles. PLoS ONE 7(10):e46944
Chen J, Tan RK, Guo XJ, Fu ZL, Wang Z, Zhang ZY, Tan XL (2015) Transcriptome analysis comparison of lipid biosynthesis in the leaves and developing seeds of Brassica napus. PLoS ONE 10(5):e0126250
Choudhury SR, Roy S, Sengupta DN (2008) Characterization of transcriptional profiles of MA-ACS1 and MA-ACO1 genes in response to ethylene, auxin, wounding, cold and different photoperiods during ripening in banana fruit. J Plant Physiol 165(18):1865–1878
Christiaens S, Van Buggenhout S, Houben K, Jamsazzadeh Kermani Z, Moelants KR, Ngouémazong ED, Van Loey A, Hendrickx ME (2016) Process-structure-function relations of pectin in food. Crit Rev Food Sci Nutr 56(6):1021–1042
Chung DW, Pruzinská A, Hörtensteiner S, Ort DR (2006) The role of pheophorbide a oxygenase expression and activity in the canola green seed problem. Plant Physiol 142(1):88–97
Du L, Song J, Forney C, Palmer LC, Fillmore S, Zhang Z (2016) Proteome changes in banana fruit peel tissue in response to ethylene and high-temperature treatments. Hortic Res 3:16012. https://doi.org/10.1038/hortres
Galpaz N, Wang Q, Menda N, Zamir D, Hirschberg J (2008) Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. Plant J 53(5):717–730
Guyer L, Hofstetter SS, Christ B, Lira BS, Rossi M, Hörtensteiner S (2014) Different mechanisms are responsible for chlorophyll dephytylation during fruit ripening and leaf senescence in tomato. Plant Physiol 166(1):44–56
Han Y, Ban Q, Hou Y, Meng K, Suo J, Rao J (2016) Isolation and characterization of two persimmon xyloglucan endotransglycosylase/hydrolase (XTH) genes that have divergent functions in cell wall modification and fruit postharvest softening. Front Plant Sci 7:624. https://doi.org/10.3389/fpls.2016.00624 (eCollection)
Jaakola L, Pirttilä AM, Halonen M, Hohtola A (2001) Isolation of high quality RNA from bilberry (Vaccinium myrtillus L.) fruit. Mol Biotechnol 19(2):201–203
Jaiswal P, Jha SN, Kaur PP, Bhardwaj R, Singh AK, Wadhawan V (2016) Prediction of textural attributes using color values of banana (Musa sapientum) during ripening. J Food Sci Technol 51(6):1179–1184
Jiang Y, Joyce DC, Macnish AJ (2000) Effect of abscisic acid on banana fruit ripening in relation to the role of ethylene. J Plant Growth Regul 19(1):106–111
Jones B, Frasse P, Olmos E, Zegzouti H, Li ZG, Latche A, Pech JC, Bouzayen M (2002) Down-regulation of DR12, an IAA-response-factor homolog, in the tomato results in a pleiotropic phenotype including dark green and blotchy ripening fruit. Plant J 32:603–613
Klee HJ, Giovannoni JJ (2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet 45:41–59
Leng P, Zhang GL, Li XX, Wang LH, Zheng ZM (2009) Cloning of 9-cis-epoxycarotenoid dioxygenase (NCED) gene encoding a key enzyme during abscisic acid (ABA) biosynthesis and ABA-regulated ethylene production in detached young persimmon calyx. Sci Bull 54(16):2830–2838
Li B, Dewey C (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform 12:323
Li JY, Tao XY, Bu JW, Ying TJ, Mao LC, Luo ZS (2017) Global transcriptome profiling analysis of ethylene-auxin interaction during tomato fruit ripening. Postharvest Biol Technol 130:28–38
Liu KD, Kang BC, Jiang H, Moore SL, Li HX, Watkins CB et al (2005) A GH3-like gene, CcGH3, isolated from Capsicum chinense L. fruit is regulated by auxin and ethylene. Plant Mol Biol 58(4):447–464
Lohani Seemi, Trivedi Prabodh K (2004) Pravendra nath.: changes in activities of cell wall hydrolases during ethylene-induced ripening in banana: effect of 1-MCP, ABA and IAA. Postharvest Biol Technol 31:119–126
Ma N, Ma X, Li A, Cao XC, Kong LG (2012) Cloning and expression analysis of wheat pheophorbide a oxygenase gene; TaPaO. Plant Mol Biol Report 30(5):1237–1245
Mittelberger C, Yalcinkaya H, Pichler C, Gasser J, Scherzer G, Erhart T, Schumacher S, Holzner B, Janik K, Robatscher P, Müller T, Kräutler B, Oberhuber M (2017) Pathogen-induced leaf chlorosis: products of chlorophyll breakdown found in degreened leaves of Phytoplasma-infected apple (malus× domestica borkh.) and apricot (Prunus armeniaca L.) trees relate to the pheophorbide a oxygenase/phyllobilin pathway. J Agric Food Chem. https://doi.org/10.1021/acs.jafc.6b05501 (Epub ahead of print)
Muday GK, Rahman A, Binder BM (2012) Auxin and ethylene: collaborators or competitors? Trends Plant Sci 17(4):181–195
Ruiz-Sola MÁ, Arbona V, Gómez-Cadenas A, Rodríguez-Concepción M, Rodríguez-Villalón A (2014) A root specific induction of carotenoid biosynthesis contributes to ABA production upon salt stress in arabidopsis. PLoS ONE 9(3):e90765
Seymour GB, Ostergaard L, Chapman NH, Knapp S, Martin C (2013) Fruit development and ripening. Annu Rev Plant Biol 64:219–241
Storey JD, Tibshirani R (2003) Statistical significance for genomewide studies. Proc Natl Acad Sci USA 100:9440–9445
Su L, Diretto G, Purgatto E, Danoun S, Zouine M, Li Z et al (2015) Carotenoid accumulation during tomato fruit ripening is modulated by the auxin-ethylene balance. BMC Plant Biol 15(1):114
Symons GM, Chua YJ, Ross JJ, Quittenden LJ, Davies NW, Reid JB (2012) Hormonal changes during non-climacteric ripening in strawberry. J Exp Bot 63:4741–4750
Trainotti L, Tadiello A, Casadoro G (2007) The involvement of auxin in the ripening of climacteric fruits comes of age: the hormone plays a role of its own and has an intense interplay with ethylene in ripening peaches. J Exp Bot 58:3299–3308
Vendrell M (1985) Effect of abscisic acid and ethephon on several parameters of ripening in banana fruit tissue. Plant Sci 40:19–24
Wang LK, Feng ZX, Wang X, Wang XW, Zhang XG (2010) DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26:136–138
Wen B, Ström A, Tasker A, West G, Tucker GA (2013) Effect of silencing the two major tomato fruit pectin methylesterase isoforms on cell wall pectin metabolism. Plant Biol (Stuttg) 15(6):1025–1032
Zhang M, Yuan B, Leng P (2009) The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot 60(6):1579–1588
Zhu MK, Chen GP, Zhou S, Tu Y, Wang Y, Dong TT et al (2014) A new tomato NAC (NAM/ATAF1/2/CUC2) transcription factor, SlNAC4, functions as a positive regulator of fruit ripening and carotenoid accumulation. Plant Cell Physiol 55(1):119–135
Acknowledgements
This research is supported by the National Natural Science Foundation of China (31772365) and the National Basic Research Program (973 program) of China (2013CB127101).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by J.-H. Liu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11738_2018_2621_MOESM1_ESM.tif
Figure S1. Volcano plot showing the number of unigenes significantly differentially expressed in response to exogenous ABA (A), IAA (B) and ABA+IAA (C). Red dots represents up regulated genes, green dots indicates down regulated genes. Supplementary material 1 (TIFF 473 kb)
11738_2018_2621_MOESM2_ESM.tif
Figure S2. H cluster (A) and Venn diagram (B) showing the distribution of differentially-expressed genes that are unique or common among treatments of ABA, IAA and ABA+IAA. Supplementary material 2 (TIFF 179 kb)
11738_2018_2621_MOESM4_ESM.xlsx
Table S1. All the enriched GO and genes information of banana peel in response to exogenous ABA and IAA. Supplementary material 4 (XLSX 30 kb)
11738_2018_2621_MOESM5_ESM.xlsx
Table S2. All the enriched KEGG and genes information of banana peel in response to exogenous ABA and IAA. Supplementary material 5 (XLSX 53 kb)
11738_2018_2621_MOESM6_ESM.xls
Table S3. Significant expressed genes involved in pigment metabolism and pectin degradation pathways. Supplementary material 6 (XLS 55 kb)
Rights and permissions
About this article
Cite this article
Lu, W., Mao, L., Chen, J. et al. Interaction of abscisic acid and auxin on gene expression involved in banana ripening. Acta Physiol Plant 40, 46 (2018). https://doi.org/10.1007/s11738-018-2621-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2621-z