Abstract
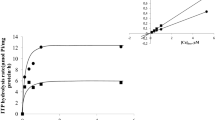
To date, it has been established that the symbiosome membrane (SM), i.e., plant-derived membrane of symbiosomes, nitrogen-fixing compartments of legume root nodules, is equipped with Ca2+-ATPase transporting Ca2+ ions through the SM from the cytosol of infected cells into the symbiosome space (SS). Earlier in the experiments on the SM vesicles isolated from broad bean root nodules some data indicating the action of the Ca2+-ATPase as ATP-driven Ca2+/H+ antiporter were obtained. In the present work performed on isolated symbiosomes from the same plant object, further evidence in favor of calcium-proton countertransport mechanism of the pump operation was obtained. These were expressed in vanadate-sensitive alkalinization of the SS coupled with Ca2+ uptake by symbiosomes catalyzed by the SM Ca2+-ATPase, stimulation of the kinetics of the latter process in the response to artificial acidification of the SS and expectable modulation of ITP-hydrolyzing activity of this enzyme caused by the variation of pH within this compartment. The above findings are discussed in the framework of the model describing the mechanism of Ca2+-ATPase operation as an ATP-driven Ca2+/H+ exchanger and on this base allow us to put forward the hypothesis about the involvement of this enzyme in symbiosome signaling in a Ca2+- and pH-dependent manner.






Similar content being viewed by others
Abbreviations
- AO:
-
Amine dye acridine orange
- HEPES:
-
N-2-hydroxyethylpiperazine-N′-ethanesulphonic acid
- BTP:
-
1,3-Bis (tris (hydroxymethyl)-methylamino) propane
- SM:
-
Symbiosome membrane
- SS:
-
Symbiosome space
- Na2EDTA:
-
Ethylene diamine tetraacetic acid disodium salt
- EGTA:
-
Ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- FCCP:
-
Carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone
- MES:
-
2-(N-morpholino) ethanesulfonic acid
References
Ames BN (1966) Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol 8:115–118
Andreev IM, Dubrovo PN, Krylova VV, Andreeva IN, Koren’kov VD, Sorokin EM, Izmailov SF (1997) Characterization of ATP-hydrolyzing and ATP-driven proton-translocating activities associated with the peribacteroid membrane from root nodules Lupinus luteus L. J Plant Physiol 151:563–569
Andreev IM, Dubrovo PN, Krylova VV, Izmailov SF (1998) Calcium uptake by symbiosomes and the peribacteroid membrane vesicles isolated from yellow lupin root nodules. J Plant Physiol 153:203–211
Andreev IM, Dubrovo PN, Krylova VV, Izmailov SF (1999) Functional identification of ATP-driven Ca2+ pump in the peribacteroid membrane of broad bean root nodules. FEBS Lett 447:49–52
Andreev IM, Krylova VV, Dubrovo PN, Izmailov SF (2005) Passive potassium transport by symbiosomes from broad bean root nodules. Plant Sci 168:1005–1010
Andreeva IN, Andreev IM, Kozharinova GM, Krylova VV, Izmailov SF (1999) Calcium stores in symbiosomes from yellow lupin root nodules. J Plant Physiol 155:357–363
Bonza MC, De Michelis MI (2011) The plant Ca2+-ATPase repertoire: biochemical features and physiological functions. Plant Biology 13:421–430
Boszek T, Lisek M, Ferenc B, Kowalski A, Stepinski D, Wiktorska M, Zylinska L (2014) Plasma membrane Ca2+-ATPase isoforms composition regulates cellular pH homeostasis in differentiating PC12 cells in a manner dependent on cytosolic Ca2+ elevations. PLoS ONE 9:e102352
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analyt Biochem 72:248–254
Carafoli E (2005) Calcium – a universal carrier of biological signals. FEBS J 272:1073–1089
Clarke VC, Loughlin PC, Day DA, Smith PMC (2015a) Transport processes of the legume symbiosome membrane. Front Plant Sci 5:699
Clarke VC, Loughlin PC, Garvin A, Chen C, Brear EM, Day DA, Smith MC (2015b) Proteomic analysis of the soybean symbiosome identifies new symbiotic proteins. Mol Cell Proteomics 14:1301–1322
Dominguez DC, Guragain M, Patrauchan M (2015) Calcium binding proteins and calcium signaling in prokaryotes. Cell Calcium 57:151–165
Felle HHE, Kondorosi E, Kondorosi A, Schultze M (1999) Elevation of the cytosolic free [Ca2+] is indispensable for the transduction of the Nod factor signal in alfalfa. Plant Physiol 121:273–280
Gazarini ML, Thomas AP, Pozzan T, Garcia CRS (2003) Calcium signaling in a low calcium environment; how the intracellular malaria parasite solves the problem. J Cell Biol 161:103–110
Inesi G, De Meis L (1989) Regulation of steady state filling in sarcoplasmic reticulum. Roles of back-inhibition, leakage and slippage of the calcium pump. J Biol Chem 265:5929–5936
Inesi G, Tadini-Buoninsegni F (2014) Ca2+/H+ exchange, luminal Ca2+ release and Ca2+/ATP coupling ratios in the sarcoplasmic reticulum ATPase. J Cell Commun Signal 8:5–11
Krylova VV, Andreev IM, Andreeva IN, Dubrovo PN, Kozharinova GM, Izmailov SF (2002) Verapamil-sensitive calcium transporter in the peribacteroid membrane of symbiosomes from Vicia faba root nodules. Russ J Plant Physiol 49:746–753
Krylova VV, Andreev IM, Zartdinova R, Izmailov SF (2013) Biochemical characteristics of the Ca2+ pumping ATPase in the peribacteroid membrane from broad bean root nodules. Protoplasma 250:531–538
Krylova VV, Zartdinova R, Andreev IM, Izmailov SF (2016) Ca2+/H+ antiport as a possible mechanism of the Ca2+- translocating ATPase functioning in vesicles of bean nodule’s symbiosome membrane. Biochemistry (Moscow) Suppl Ser A Membr Cell Biol 10:218–222
Leborgne-Castel N, Bouhidel K (2014) Plasma membrane protein trafficking in plant-microbe interactions: a plant cell point of view. Front Plant Sci 5:735
Liu J, Miller SS, Graham M, Bucciarelly B, Catalano CM, Sherrier DJ, Samac DA, Ivashuta S, Fedorova M, Matsumoto P, Gantt JS, Vance CP (2006) Recruitment of novel calcium-binding proteins for root nodule symbiosis in Medicago trunicatula. Plant Physiol 141:167–177
Oke V, Long SR (1999) Bacteroid formation in the Rhizobium-legume symbiosis. Cur Opin Microbiol 2:641–646
Oldroyd GE, Downie JA (2004) Calcium, kinases and nodulation signaling in legumes. Nat Rev Mol Cell Biol 5:566–576
Palmgren MG (1991) Acridine orange as a probe for measuring pH gradients across membranes: mechanism and limitations. Anal Biochem 192:316–321
Perez-Cordones MC, Lugo MR, Winkler M, Cervino V, Benaim G (2009) Diacylglycerol regulates the plasma membrane calcium pump from human erythrocytes by direct inter-action. Arch Biochem Biophys 489:55–61
Plieth C (2005) Calcium: just another regulator in the machinery of life? Ann Bot 96:1–8
Udvardi MK, Day DA (1997) Metabolite transport across symbiotic membranes of legume nodules. Annu Rev Plant Physiol Plant Mol Biol 48:493–523
Udvardi MK, Pool PS (2013) Transport and metabolism in legume-rhizobia symbioses. Annu Rev Plant Biol 64:781–805
Verhaert J, Vanderleyden J, Michiels J (2005) Bacterial endocytic systems in plants and animals: Ca2+ as a common theme? Crit Rev Plant Sci 24:283–308
Zhai J, Xu H, Cong X, Deng Y, Xia Z, Huang X, Hao G, Jiang X (2013) Ca2+/H+ exchange in the plasma membrane of Arabidopsis thaliana leaves. Acta Physiol Plant 35:161–173
Acknowledgements
This work was supported by a Grant No. 15-04-02451 from Russian Foundation for Basic Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. A. Kleczkowski.
Rights and permissions
About this article
Cite this article
Krylova, V.V., Andreev, I.M., Zartdinova, R. et al. Ca2+-ATPase in the symbiosome membrane from broad bean root nodules: further evidence for its functioning as ATP-driven Ca2+/H+ exchanger. Acta Physiol Plant 39, 247 (2017). https://doi.org/10.1007/s11738-017-2546-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-017-2546-y