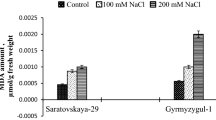
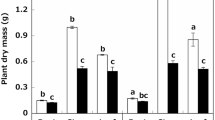
Abstract
To examine the impact of long-term salinity on triticale, two salt-tolerant (ET-84-15 and ET-86-9) and two salt-sensitive (ET-85-17 and Jouvanilo) genotypes were grown in a sand culture containing Hoagland solution with 200 mM NaCl. Lipid peroxidation (LPO), hydrogen peroxide (H2O2), photosynthetic parameters, and antioxidant enzymes including catalase (CAT), guaiacol peroxidase (GPOD), superoxide dismutase (SOD), and ascorbate peroxidase (APX) were analyzed at the late tillering (LT) and flowering (FL) stages. A substantial reduction was found in the net photosynthetic rate, stomatal conductance, and transpiration rate of the triticale genotypes due to salt stress, with a more noticeable decline in the sensitive ones. Salt-treated plants indicated the presence of high amounts of H2O2 and LPO with a subsequent increase in the activities of the enzymes’ SOD, CAT, and GPOD in comparison with the control treatment. Conversely, APX activities remained unaltered or decreased slightly by salt stress. The salt-tolerant genotypes exhibited lower H2O2 and LPO, and displayed increased activities of the enzymes participating in the reactive oxygen scavenging system except for APX. The activities of the antioxidant enzymes under both the saline and non-saline conditions were found to be higher at the FL stage than at the LT one. This may explain partly the reason for why triticale is more tolerant at the FL stage. These results clearly demonstrate that the activation of SOD, CAT, and GPOD could contribute to the salt-stress tolerance in triticale.





Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Method Enzymol 105:121–126
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24:1337–1344
Asada K, Takahashi MT (1987) Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osmond CD, Arntzen CJ (eds) Photoinhibition. Elsevier Science publishers, Amsterdam, pp 227–287
Beauchamp C, Fridovich I (1971) Superoxide dismutase, improved assays and applicable to acrylamide gels. Anal Biochem 44:276–287
Bole JB, Wells SA (1979) Dryland soil salinity: effect on the yield and yield components of 6-row barley, 2-row barley, wheat and oats. Can J Soil Sci 59:11–17
Bor M, Özdemir F, Türkan I (2003) The effect of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritima L. Plant Sci 164:77–84
Brugnoli E, Lauteri M (1991) Effects of salinity on stomatal conductance, photosynthetic capacity, and carbon isotope discrimination of salt tolerant (Gossypium hirsutum L.) and salt-sensitive (Phaseolus vulgaris L.) C3 non-halophytes. Plant Physiol 95:628–635
Chance B, Maehly AC (1955) Assay of catalase and peroxidases. Methods Enzymol 2:764–775
Dąbrowska GR, Kata AL, Goc A, Szechynska-Hebda M, Skrzypek ED (2007) Characteristics of the plant ascorbate peroxidase family. Acta Biol Cracov Bot 49:7–17
Desingh R, Kanagaraj G (2007) Influence of salinity stress on photosynthesis and antioxidative systems two cotton varieties. Gen Appl plant Physiol 33:221–234
El-Hendawy S (2004) Salinity tolerance in Egyptian spring wheat genotypes. Ph.D. Thesis, Department für Pflanzenwissenschaften Technische Univ. München, Germany
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions in order to enhance photosynthesis. Plant Physiol 155:93–100
Foyer CH, Descourvieres P, Kunert KJ (1994) Protection against oxygen radicals: an important defence mechanism studied in transgenic plants. Plant Cell Environ 17:507–523
Ghassemi F, Jakerman AJ, Nix HA (1995) Salinization of land water resources. CAB International, Wallingford
Ghorbani Javid M, Sorooshzadeh A, Moradi F, Modarres Sanavy SAM, Allahdadi I (2011) The role of phytohormones in alleviating salt stress in crop plants. Aust J Crop Sci 5:726–734
Gill KS, Dutt SK (1982) Effect of salinity on stomatal number, size and in barley genotypes. Physiol Plant 24:266–269
Gorham J (1990) Salt tolerance in the triticeae: ion discrimination in rye and triticale. J Exp Bot 41:609–614
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
James RA, Rivelli AR, Munns R, Von Caemmerer S (2002) Factors affecting CO2 assimilation, leaf injury and growth in salt-stressed durum wheat. Funct Plant Biol 29:1393–1403
Jessop R (1996) Stress tolerance in newer triticales compared to other cereals. In: Guedes-Pinto H, Darvey N, Carnide V (eds) Triticale: today and tomorrow. Springer, Netherlands, pp 419–427
Jiang Q, Roche D, Monaco TA, Durham S (2006) Gas exchange, chlorophyll fluorescence parameters and carbon isotope discrimination of 14 barley genetic lines in response to salinity. Field Crop Res 96:269–278
Kendall AC, Keys AJ, Turner JC, Lea PJ, Miflin BJ (1983) The isolation and characterization of a catalase-deficient mutant of barley. Planta 159:505–511
Kholova J, Sairam RK, Meena RC (2010) Osmolytes and metal ions accumulation, oxidative stress and antioxidant enzymes activity as determinants of salinity stress tolerance in maize genotypes. Acta Physiol Plant 32:477–486
Kiani-Pouya A, Rasouli F (2014) The potential of leaf chlorophyll content to screen bread-wheat genotypes in saline condition. Photosynthetica 52:288–300
Koebner RD, Martin P (1996) High levels of salt tolerance revealed in triticale. In: Guedes-Pinto H, Darvey N, Carnide V (eds) Triticale: today and tomorrow. Springer, Netherlands, pp 429–436
Maas EV, Poss JA (1989) Salt sensitivity of wheat at different growth stages. Irrig Sci 10:29–40
Maksimovic JD, Zhang J, Zeng F, Živanovic BD, Shabala L, Zhou M, Shabala S (2013) Linking oxidative and salinity stress tolerance in barley: can root antioxidant enzyme activity be used as a measure of stress tolerance? Plant Soil 365:141–155
Mansfield TA, Hetherington AM, Atkinson CJ (1990) Some current aspects of stomatal physiology. Annu Rev Plant Physiol Plant Mol Biol 41:55–75
Moradi F, Ismail AM (2007) Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann Bot 99:1161–1173
Morant-Manceau A, Pradier E, Tremblin G (2004) Osmotic adjustment, gas exchanges and chlorophyll fluorescence of a hexaploid triticale and its parental species under salt stress. J Plant Physiol 161:25–33
Munns R, James RA (2003) Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant Soil 253:201–218
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Poljsak B, Šuput D, Milisav I (2013) Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants Oxid Med Cell Longev. doi:10.1155/2013/956792
Prakash V, Mishra PK, Mishra M (2009) Screening of medicinal plants extract for antioxidant activity. J Med Plants Res 3:608–612
Reddy PS, Ramanjulu S, Sudhakar C, Veeranjaneyulu K (1998) Differential sensitivity of stomatal and non-stomatal components to NaCl or Na2SO4 salinity in horsegram, Macrotyloma uniflorum (Lam). Photosynthetica 35:99–105
Reynolds MP, Ortiz-Monasterio JI, McNab A (2001) Application of physiology in wheat breeding. CIMMYT, Mexico, D.F.
Richards RA (2000) Selectable traits to increase crop photosynthesis and yield of grain crops. J Exp Bot 51:447–458
Richards RA, Dennett CW, Qualset CO, Epstein E, Norlyn JD, Winslow MD (1987) Variation in yield of grain and biomass in wheat, barley, and triticale in a salt-affected field. Field Crop Res 15:277–287
Robinson MF, Véry A, Sanders D, Mansfield TA (1997) How can stomata contribute to salt tolerance? Ann Bot 80:387–393
Royo A, Aragues R (1999) Salinity-yield response functions of barley genotypes assessed with a triple line source sprinkler system. Plant Soil 209:9–20
Sairam RK, Rao KV, Srivastava GC (2002) Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci 163:1037–1046
Salehi M, Arzani A (2014) Evaluation of triticale genotypes for salt tolerance using physiological traits. Emir J Food Agric 26:277–283
Salim M (1988) Growth and ionic relations of six triticale cultivars as affected by salinity. Biol Plant 30:294–299
SAS Institute Inc. (2003) SAS/STAT User’s Guide, version 9.1.3. SAS Institute Inc, Cary, N.C.
Scandalios JG (1993) Oxygen stress and superoxide dismutase. Plant Physiol 101:7–12
Sevanian A (1988) Lipid peroxidation in biological systems. Am Oil Chem Soc, USA
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. doi:10.1155/2012/217037
Sudhakar C, Lakshmi A, Giridarakumar S (2001) Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci 161:613–619
Swapna TS (2003) Salt stress induced changes on enzyme activities during different developmental stages of rice. Indian J Biotechnol 2:251–258
Tseng MJ, Liu CW, Yiu JC (2007) Enhanced tolerance to sulfur dioxide and salt stress of transgenic Chinese cabbage plants expressing both superoxide dismutase and catalase in chloroplasts. Plant Physiol Biochem 45:822–833
Varga B, Janda T, Laszlo E, Veisz O (2012) Influence of abiotic stresses on the antioxidant enzyme activity of cereals. Acta Physiol Plant 34:849–858
Acknowledgments
This project was financially supported by the Agriculture Organization in the Fars province, and Ministry of Agriculture in Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Zwiazek.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kiani-Pouya, A. Changes in activities of antioxidant enzymes and photosynthetic attributes in triticale (×Triticosecale Wittmack) genotypes in response to long-term salt stress at two distinct growth stages. Acta Physiol Plant 37, 72 (2015). https://doi.org/10.1007/s11738-015-1819-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1819-6