Abstract
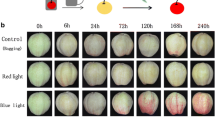
Anthocyanins impart red, purple and violet colour to many flowers and fruits, mainly to attract pollinators and seed dispersers, but their function and biosynthetic regulation in foliages of several plants are less studied. The red and green forma of Ocimum tenuiflorum differ in anthocyanin accumulation in leaves and provide an excellent system for exploring the course of its regulation. It was observed that red forma gradually changed to green upon transfer to a particular greenhouse with limited transmission of ultraviolet light (both UV-B and UV-A). The sequential monitoring of anthocyanin content confirmed positive correlation between visible and ultraviolet light intensity with leaf colour and antioxidant activities. An ultra-performance liquid chromatography method of <3.5 min was developed for rapid and precise quantification of anthocyanidins. Expressions of PAL, CHS and CHI were down-regulated by low light in both forma. The F3H and F3′H genes had reduced expression in both forma and were supported by reduced levels of cyanidin in red forma plants within greenhouse. The expression of late biosynthetic genes, DFR and LDOX, also plummeted within the greenhouse. The regulatory transcription factors bHLH and WD40 were severely down-regulated within the greenhouse suggesting that bHLH and WD40 control the expression of F3′H, DFR and LDOX to regulate the biosynthesis of anthocyanin pigments in leaves of O. tenuiflorum, whereas the expression of Myb remained almost unaffected.





Similar content being viewed by others
Abbreviations
- bHLH:
-
Basic helix loop helix
- CHI:
-
Chalcone isomerase
- CHS:
-
Chalcone synthase
- CIE:
-
Commission Internationale d’Eclairage
- DFR:
-
Dihydro flavonol reductase
- F3H:
-
Flavonone 3 hydroxylase
- F3′H:
-
Flavonone 3′ hydroxylase
- FRAP:
-
Ferric reducing antioxidant power assay
- LATAMOS:
-
Land surface atmosphere and micrometeorological observational system
- LDOX:
-
Leucoanthocyanidin dioxygenase
- MBW:
-
Myb-bHLH-WD40
- MSA:
-
Multiple sequence alignment
- PAL:
-
Phenylalanine ammonia lyase
- qPCR:
-
Quantitative real-time polymerase chain reaction
- Q-Tof:
-
Quadrupole-time-of-flight
- UPLC:
-
Ultra-performance liquid chromatography
References
Albert NW, Lewis DH, Zhang H, Irving LJ, Jameson PE, Davies KM (2009) Light-induced vegetative anthocyanin pigmentation in Petunia. J Exp Bot 60:2191–2202. doi:10.1093/jxb/erp097
Allan AC, Hellens RP, Laing WA (2008) MYB transcription factors that colour our fruit. Trends Plant Sci 13:99–102. doi:10.1016/j.tplants.2007.11.012
An XH, Tian Y, Chen KQ, Wang XF, Hao YJ (2012) The apple WD40 protein MdTTG1 interacts with bHLH but not MYB protein to regulate anthocyanin accumulation. J Plant Physiol 169:710–717. doi:10.1016/j.jplph.2012.01.015
Benzie IEF, Strain JJ (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299:15–27. doi:10.1016/S0076-6879(99)99005-5
Chiu LW, Zhou X, Burke S, Wu X, Prior RL, Li L (2010) The purple cauliflower arises from activation of a MYB transcription factor. Plant Physiol 154:1470–1480. doi:10.1104/pp.110.164160
Dudonné S, Vitrac X, Coutière P, Wollez M, Mérillon J-M (2009) Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD and ORAC assays. J Agric Food Chem 57:1768–1774. doi:10.1021/jf803011r
Felsenstein J (2002) Quantitative characters, phylogenies, and morphometrics. In: MacLeod N (ed) Morphology, shape and phylogenetics. Systematics association, vol 64, pp 27–44. doi:10.1201/9780203165171.ch3
Gollop R, Farhi S, Perl A (2001) Regulation of leucoanthocyanidin dioxygenase gene expression in Vitis vinifera. Plant Sci 161:579–588. doi:10.1016/S0168-9452(01)00445-9
Gollop R, Even S, Colova-Tsolova V, Perl A (2002) Expression of the grape dihydroflavonol reductase gene and analysis of its promoter region. J Exp Bot 53:1397–1409. doi:10.1093/jexbot/53.373.1397
Gong ZZ, Yamazaki M, Sugiyama M, Tanaka Y, Saito K (1997) Cloning and molecular analysis of structural genes involved in anthocyanin biosynthesis and expressed in a forma-specific manner in Perilla frutescens. Plant Mol Biol 35:915–927. doi:10.1023/A:1005959203396
Gong ZZ, Yamazaki M, Saito K (1999a) A light inducible Myb-like gene that is specifically expressed in red Perilla frutescens and presumably acts as a determining factor of the anthocyanin forma. Mol Gen Genet 262:65–72. doi:10.1007/PL00008639
Gong ZZ, Yamagishi E, Yamazaki M, Saito K (1999b) A constitutively expressed Myc-like gene involved in anthocyanin biosynthesis from Perilla frutescens: molecular characterization, heterologous expression in transgenic plants and transactivation in yeast cells. Plant Mol Biol 41:33–44. doi:10.1023/A:1006237529040
Gould KS (2004) Nature’s Swiss army knife: the diverse protective roles of anthocyanins in leaves. J Biomed Biotechnol 5:314–320. doi:10.1155/S1110724304406147
Grotewold E (2006) The genetics and biochemistry of floral pigments. Annu Rev Plant Biol 57:761–780. doi:10.1146/annurev.arplant.57.032905.105248
Hichri I, Barrieu F, Bogs J, Kappel C, Delrot S, Lauvergeat V (2011) Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J Exp Bot 62:2465–2483. doi:10.1093/jxb/erq442
Holton TA, Cornish EC (1995) Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 7:1071–1083. doi:10.1105/tpc.7.7.1071
Hosseinian FS, Li W, Beta T (2008) Measurement of anthocyanins and other phytochemicals in purple wheat. Food Chem 109:916–924. doi:10.1016/j.foodchem.2007.12.083
Hurtado NH, Morales AL, Gonzalez-Miret L, Escudero-Gilete L, Heredia FJ (2009) Colour, pH stability and antioxidant activity of anthocyanin rutinosides isolated from tamarillo fruit (Solanum betaceum Cav.). Food Chem 117:88–93. doi:10.1016/j.foodchem.2009.03.081
Kayesh E, Shangguan L, Korir NK, Sun X, Bikish N, Zhang Y, Han J, Song C, Cheng ZM, Fang J (2013) Fruit skin color and the role of anthocyanin. Acta Physiol Plant 35:2879–2890
Kobayashi H, Suzuki S, Tanzawa F, Takayanagi T (2009) Low expression of flavonoid 3′5′-hydroxylase (F3′5′H) associated with cyanidin-based anthocyanins in grape leaf. Am J Enol Vitic 60:362–367
Laitinen RAE, Ainasoja M, Borholm SK, Teeri TH, Elomaa P (2008) Identification of target genes for a MYB-type anthocyanin regulator in Gerbera hybrida. J Exp Bot 59:3691–3703. doi:10.1093/jxb/ern216
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal X and Clustal W Version 2.0. Bioinformatics 23:2947–2948
Neill SO, Gould KS (1999) Optical properties of leaves in relation to anthocyanin concentration and distribution. Can J Bot 77:1777–1782. doi:10.1139/cjb-77-12-1777
Neill SO, Gould KS, Kilmartin PA, Mitchell KA, Markham KR (2002) Antioxidant activities of red versus green leaves in Elatostema rugosum. Plant, Cell Environ 25:539–548. doi:10.1046/j.1365-3040.2002.00837.x
Niu SS, Xu CJ, Zhang WS, Zhang B, Li X, Wang KL, Ferguson IB, Allan AC, Chen KS (2010) Coordinated regulation of anthocyanin biosynthesis in Chinese bayberry (Myrica rubra) fruit by a R2R3 MYB transcription factor. Planta 231:887–899. doi:10.1007/s00425-009-1095-z
Park NI, Xiaohua L, Suzuki T, Kim SJ, Woo SH, Park CH, Park SU (2011) Differential expression of anthocyanin biosynthetic genes and anthocyanin accumulation in Tartary buckwheat cultivars ‘Hokkai T8’ and ‘Hokkai T10’. J Agric Food Chem. doi:10.1021/jf200020b
Poustka F, Irani NG, Feller A, Lu Y, Poureel L, Frame K, Grotewold E (2007) A trafficking pathway for anthocyanins overlaps with the endoplasmic reticulum -to- vacuole protein-sorting route in Arabidopsis and contributes to the formation of vacuolar inclusions. Plant Physiol 145:1323–1335. doi:10.1104/pp.107.105064
Ramsay NA, Glover BJ (2005) MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci 10:63–70. doi:10.1016/j.tplants.2004.12.011
Renu IK, Haque I, Kumar M, Poddar R, Bandopadhyay R, Rai A, Mukhopadhyay K (2014) Characterization and functional analysis of eugenol O-methyltransferase gene reveal metabolite shifts, chemotype specific differential expression and developmental regulation in Ocimum tenuiflorum L. Mol Biol Rep 41:1857–1870. doi:10.1007/s11033-014-3035-7
Rieu I, Powers SJ (2009) Real-time quantitative RT-PCR: design, calculations and statistics. Plant Cell 21:1031–1033. doi:10.1105/tpc.109.066001
Rowan DD, Cao M, Wang KL, Cooney JM, Jensen DJ, Austin PT, Hunt MB, Norling C, Hellens RP, Schaffer RJ, Allan AC (2009) Environmental regulation of leaf colour in red 35S:PAP1 Arabidopsis thaliana. New Phytol 182:102–115. doi:10.1111/j.1469-8137.2008.02737.x
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Saito K, Yamazaki M (2002) Biochemistry and molecular biology of the late-stage of biosynthesis of anthocyanin: lessons from Perilla frutescens as a model plant. New Phytol 155:9–23. doi:10.1046/j.1469-8137.2002.00440.x
Sakaguchi Y, Ozaki Y, Miyajima I, Yamaguchi M, Fukui Y, Iwasa K, Motoki S, Suzuki T, Okubo H (2008) Major anthocyanins from purple asparagus (Asparagus officinalis). Phytochemistry 69:1763–1766. doi:10.1016/j.phytochem.2008.02.021
Singh D, Bhaganagare G, Bandopadhyay R, Prabhu KV, Gupta PK, Mukhopadhyay K (2012) Targeted spatio-temporal expression based characterization of state of infection and time-point of maximum defense in wheat NILs during leaf rust infection. Mol Biol Rep 39:9373–9382
Sompornpailin K, Makita Y, Yamazaki M, Saito K (2002) A WD-repeat-containing putative regulatory protein in anthocyanin biosynthesis in Perilla frutescens. Plant Mol Biol 50:485–495. doi:10.1023/A:1019850921627
Vyas P, Chaudhary B, Mukhopadhyay K, Bandopadhyay R (2009) Anthocyanins: looking beyond colors. In: Bhowmick PK, Basu SK, Goyal A (eds) Advances in biotechnology. Bentham Science Publishers Ltd., Illinois, pp 152–184
Yamazaki M, Makita Y, Springob K, Saito K (2003a) Regulatory mechanisms for anthocyanin biosynthesis in chemotypes of Perilla frutescens var. crispa. Biochem Eng J 14:191–197. doi:10.1016/S1369-703X(02)00222-X
Yamazaki M, Nakajima J, Yamanishi M, Sugiyama M, Makita Y, Springob K, Awazuhara M, Saito K (2003b) Metabolomics and differential gene expression in anthocyanin chemo-varietal forms of Perilla frutescens. Phytochemistry 62:987–995. doi:10.1016/S0031-9422(02)00721-5
Yuan Y, Chiu LW, Li L (2009) Transcriptional regulation of anthocyanin biosynthesis in red cabbage. Planta 230:1141–1153. doi:10.1007/s00425-009-1013-4
Zhang D, Qian M, Yu B, Teng Y (2013) Effect of fruit maturity on UV-B-induced post-harvest anthocyanin accumulation in red Chinese sand pear. Acta Physiol Plant 35:2857–2866
Acknowledgments
We gratefully acknowledge Dr. Manoj Kumar of Department of Applied Mathematics, BIT-Mesra for providing the LATAMOS data, Mr. Sanjay Swain, Mr. Ashwani Singh and Mr. Dharmendra Singh of BIT-Mesra for excellent technical assistance. The work was supported, in part, by University Grants Commission of India [34-275\2008 SR], Ministry of Food Processing Industries, India [47/MFPI/R&D/2006/517], and Infrastructure Development Fund by Department of Agriculture, Government of Jharkhand [5/B.K.V/Misc/12/2001]. Fellowships were provided to PV by BIT-Mesra and IH by CSIR [9/554 (13) 2007-EMR-I].
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Kovacik.
P. Vyas and I. Haque have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vyas, P., Haque, I., Kumar, M. et al. Photocontrol of differential gene expression and alterations in foliar anthocyanin accumulation: a comparative study using red and green forma Ocimum tenuiflorum . Acta Physiol Plant 36, 2091–2102 (2014). https://doi.org/10.1007/s11738-014-1586-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-014-1586-9