Abstract
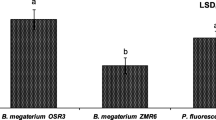
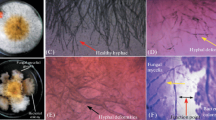
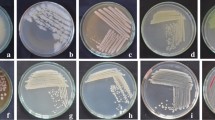
This study aimed at investigating the efficacy of a PGPR strain, Bacillus subtilis 21-1 (BS21-1), under two different soil conditions for plant growth promotion and disease suppression. BS21-1 treatment significantly (P < 0.05) promoted plant growth as measured by plant height and leaf width and increased the seed germination rate in organic soil (OS) compared with seed bed soil (SBS). In Chinese cabbage and lettuce, soft rot disease was reduced to 45 and 23.5 %, respectively, by BS21-1, and to 33 and 52.5 %, respectively, by benzo-(1,2,3)-thidiazole-7-carbothioic acid S-methyl ester (BTH) treatments in OS compared with SBS. These levels of disease suppression were greater than those found in the water-treated control upon pathogen challenge. There was a greater reduction of anthracnose lesions on the leaves of cucumber plants treated with BS21-1 and BTH in OS when compared with SBS. Botrytis rot disease in tomato caused by Botrytis cinerea was drastically reduced to 2 % in OS and 4 % in SBS by BS21-1 treatment. In the four plants studied, there was increased disease suppression in OS compared with SBS. Upon treatment with BS21-1, there was an increased expression of the PR-1a gene by β-gulcuronidase (GUS) activity in tobacco (Nicotiana tabacum L. cv. Xanthi-nc) plants in OS compared with SBS, which indicates a possible role of the SA pathway in BS21-1 mediated plant protection. Thus, the isolate BS21-1 could effectively be used as one of the biocontrol agents for disease suppression in four vegetable crops through systemic resistance and for plant growth promotion.






Similar content being viewed by others
References
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Ann Rev Plant Biol 57:233–266
Banerjee S, Palit R, Sengupta C, Standing D (2010) Stress induced phosphate solubilization by Arthrobacter sp. and Bacillus sp. isolated from tomato rhizosphere. Austr J Crop Sci 4:378–383
Bashan Y, Holguin G (1997) Azospirillum-plant relationship: environmental and physiological advances. Can J Microbiol 43:103–121
Bent E (2006) Induced systemic resistance mediated by plant growth-promoting rhizobacteria (PGPR) and fungi (PGPF). In: Tuzun S, Bent E (eds) Multigenic and induced systemic resistance in plants. Springer, New York, pp 225–258
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28:1327–1350
Catinot JA, Buchala AE, Mansour Metraux JP (2008) Salicylic acid production in response to biotic and abiotic stress depends on isochorismate in Nicotiana benthamiana. FEBS Lett 582:473–478
Chebotar VK, Asis CA Jr, Akao S (2001) Production of growth-promoting substances and high colonization ability of rhizobacteria enhance the nitrogen fixation of soybean when coinoculated with Bradyrhizobium japonicum. Biol Fertil Soils 34:427–432
Chun J, Lee JH, Jung Y, Kim M, Kim BK, Lim YW (2007) EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int Syst Evol Microbiol 57:2259–2261
Dashti N, Zhang F, Hynes R, Smith DL (1998) Plant growth promoting rhizobacteria accelerate nodulation and increase nitrogen fixation activity by field grown soybean [Glycine max (L.) Merr.] under short season conditions. Plant Soil 200:205–213
Felsenstein J (1981) Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol 17:368–376
Gupta A, Gopal M, Tilak KV (2000) Mechanism of plant growth promotion by rhizobacteria. Indian J Exp Biol 38:856–862
Heil M (1999) Systemic acquired resistance: available information and open ecological questions. J Ecol 87:341–346
Heil M, Hilpert A, Kaiser W, Linsenmair KE (2000) Reduced growth and seed set following chemical induction of pathogen defense: does systemic acquired resistance (SAR) incur allocation costs? J Ecol 88:645–654
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5:387–405
Jeun YC, Lee YJ, Bae YS (2004) Rhizobacteria-mediated Induced Systemic Resistance in Cucumber Plants against Anthracnose Disease Caused by Colletotrichum orbiculare. Plant Pathol J 20(3):172–176
Kavino M, Harish S, Kumar N, Saravanakumar D, Samiyappan R (2010) Effect of chitinolytic PGPR on growth, yield and physiological attributes of banana (Musa spp.) under field conditions. Appl Soil Ecol 45:71–77
Kloepper JW, Zablotowicz RM, Tipping EM, Lifshitz R (1991) Plant growth promotion mediated by bacterial rhizosphere colonizers. In: Keister DL, Cregan PB (eds) The rhizosphere and plant growth. Kluwer Academic Publishing, Dordrecht, pp 315–326
Kloepper JW, Ryu CM, Zhang S (2004) Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 94:1259–1266
Landa BB, Navas-Cortés JA, Jiménez-Diaz RM (2004) Influence of temperature on plant–rhizobacteria interactions related to biocontrol potential for suppression of fusarium wilt of chickpea. Plant Pathol 53:341–352
Mendoza Garcia RA, Martijn ten Hoopen G, Kass DCJ, Sanchez Garita VA, Krauss U (2003) Evaluation of mycoparasites as biocontrol agents of Rosellinia root rot in cocoa. Biol Control 27:210–227
Montealegre JR, Reyes Perez LM, Herrera R, Silva P, Besoain X (2003) Selection of bio-antagonistic bacteria to be used in biological control of Rhizoctonia solani in tomato. Electron J Biotechnol 6:115–127
Ongena M, Jacques P (2007) Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol 16:114–125
Park KS, Kloepper JW (2000) Activation of PR-1a promoter by rhizobacteria which induce systemic resistance in tobacco against Pseudomonas syringae pv. tabaci. Biol Control 18:2–9
Patten CL, Glick BR (2002) Role of Pseudomonas putida indole acetic acid in development of the host plant root system. Appl Environ Microbiol 68:3795–3801
Pikovskaya RI (1948) Mobilization of phosphorus in soil in connection with the vital activity of some microbial species. Mikrobiologia 17:362–370
Raupach GS, Liu I, Murphy IF, Tuzun S, Kloepper JW (1996) Induced systemic resistance in cucumber and tomato against cucumber mosaic virus using plant growth promoting rhizobacteria (PGPR). Plant Dis 80:891–894
Reitz M, Rudolph K, Schroder K, Hoffmann-Hergarten S, Hallmann J, Sikora RA (2000) Lipopolysaccharides of rhizobium etli strain G12 act in potato roots as an inducing agent of systemic resistance to infection by the cyst nematode Globodera pallida. Appl Environ Microbiol 66:3515–3518
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Ryu CM, Mohamed AF, Chia-Hui H, Munagala SR, Joseph WK, Paul WP (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sang MK, Kim KD (2011) Biocontrol activity and primed systemic resistance by compost water extracts against anthracnoses of pepper and cucumber. Phytopathology 101:732–740
Sang MK, Kim JG, Kim KD (2010) Biocontrol activity and induction of systemic resistance in pepper by compost water extracts against Phytophthora capisci. Phytopathology 100:774–783
Sang MK, Kim JG, Kim BS, Kim KD (2011) Root treatment with rhizobacteria antagonistic to phytophthora blight affects anthracnose occurrence, ripening, and yield of pepper fruit in the plastic house and field. Phytopathology 101:666–678
SAS Institute (1995) JMP statistics and graphics guide, version 3. SAS Institute, Cary, pp 65–95
Schneider M, Schweizer P, Meuwly P, Metraux JP (1996) Systemic acquired resistance in plants. In: Jeon KW (ed) International review of cytology, vol 168. Academic Press, San Diego, pp 303–340
Tamura K, Dudley J, Nei M, Kumar S (2007) Molecular evolutionary genetics and analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thakuria D, Talukdar NC, Goswami C, Hazarika S, Boro RC, Khan MR (2004) Characterization and screening of bacteria from rhizosphere of rice grown in acidic soils of Assam. Curr Sci 86:978–985
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Van Loon LC, Glick GR (2004) Increased plant fitness by rhizobacteria. In: Sandermann H (ed) Molecular ecotoxicology of plants, vol 170. Springer, Berlin, pp 177–205
Venkatesan P (2008) Induced disease resistance elicited by acibenzolar-S-methyl and plant growth-promoting rhizobacteria in tobacco (Nicotiana tabacum L.). Ph.D thesis submitted to Faculty of the Virginia Polytechnic Institute and State University Blacksburg, Virginia pp 188
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Wei G, Kloepper JW, Tuzun S (1991) Induction of systemic resistance of cucumber to Colletotriichum orbiculare by select strains of plant growth-promoting rhizobacteria. Phytopathology 81:1508–1512
Zhang S, White TL, Martinez MC, McInroy JA, Kloepper JW, Klassen W (2010) Evaluation of plant growth-promoting rhizobacteria for control of Phytophthora blight on squash under greenhouse conditions. Biol Control 53:129–135
Acknowledgments
The study was supported in part by a grant (Project code: PJ008652) from National Academy of Agricultural Sciences (NAAS), RDA, South Korea, 441-707.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. J. Reigosa.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, SW., Lee, SH., Balaraju, K. et al. Growth promotion and induced disease suppression of four vegetable crops by a selected plant growth-promoting rhizobacteria (PGPR) strain Bacillus subtilis 21-1 under two different soil conditions. Acta Physiol Plant 36, 1353–1362 (2014). https://doi.org/10.1007/s11738-014-1514-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-014-1514-z